Table of Contents
The name of the relevant alkyl group is usually followed by the word “alcohol,” e.g., methyl alcohol, ethyl alcohol. Depending on whether the hydroxyl group is attached to the end or middle carbon on the straight propane chain, propyl alcohol is either n-propyl alcohol or isopropyl alcohol. If another group on the molecule takes precedence, the alcohol moiety is commonly designated with the “hydroxy-” prefix, as mentioned under systematic naming. Secondary alcohols have the formula RR’CHOH, with 2-propanol (R=R’=CH3) being the most basic.
A Brief Outline
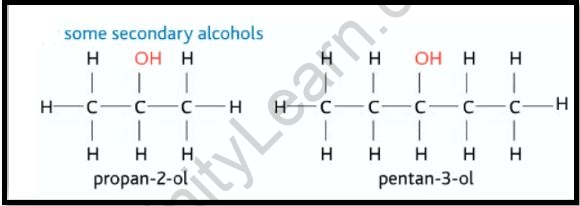
Secondary alcohols are formed when the carbon atom of the hydroxyl group is linked to two alkyl groups on either side. Secondary alcohol has two alkyl groups that can be structurally identical or even different. Propane-2-ol, butan-2-ol, and pen-3-ol are examples of secondary alcohols. Distinct chemical techniques are used to make various forms of alcohol. The kind of alcohol, the existence of functional groups, and other factors influence the preparation processes. These three main processes can be used to make alcohol in general. The following are the details.
- Distillation of Fermented Grains or Fruit Mixes – The distillation of fermented grains and fruit mixtures is common. To make or produce alcohol, grains, fruits, or vegetables are utilized in the fermentation process, which involves yeast or bacteria performing chemical reactions with sugars in food, resulting in the production of ethanol and carbon dioxide as by-products.
- Chemical Modification of Fossil Fuels, such as Oil, Natural Gas, or Coal – This process yields industrial alcohol.
- Methanol or wood alcohol is produced by combining hydrogen and carbon monoxide chemically.
Simple alcohols have low acute toxicity when it comes to acute toxicity. Several millilitre doses are tolerated. The LD50 for pentanols, hexanols, octanols, and longer alcohols is 2–5 g/kg (rats, oral). Methanol and ethanol are less harmful in the short term. Alcohols are all mild irritants to the skin. The presence of ethanol, which has a higher affinity for liver alcohol dehydrogenase, affects the metabolism of methanol (and ethylene glycol). Methanol will be eliminated intact in urine in this manner.
Important concepts
Secondary alcohol examples and group
Isopropyl alcohol
Propylene is indirectly hydrated to produce isopropyl alcohol (2-propanol) (CH2CHCH3). Isopropyl alcohol is extensively used as a skin rubbing alcohol and as an industrial solvent. Although isopropyl alcohol is more harmful than ethanol, it has a less drying effect on the skin and is neither regulated nor taxed in the United States. It’s a frequent ingredient in antiseptics, disinfectants, and detergents, and it’s utilized in the production of a wide range of industrial and household chemicals.
Water, ethanol, and chloroform are largely miscible with isopropyl liquor. Ethylcellulose, polyvinyl butyral, an assortment of oils, alkaloids, gums, and normal gums are totally broken down by it. Isopropyl liquor isn’t miscible with salt arrangements, in contrast to ethanol or methanol, and can be confined from fluid frameworks by adding a salt like sodium chloride. Salting out is a term used to portray the partition of amassed isopropyl liquor into a particular layer.
Isopropyl alcohol can dissolve a wide range of non-polar compounds. It also evaporates quickly, leaves virtually little oil traces, and is typically non when contrasted to other solvents. As a result, it’s often employed as a solvent and cleaning fluid, especially for dissolving oils. Together with ethanol, n-butanol, and methanol, it is an alcohol solvent.
Butanol secondary alcohol
The chemical molecule 2-butanol, often known as sec-butanol, has the formula CH3CH(OH)CH2CH3. This secondary alcohol is a flammable, colourless liquid that is entirely miscible with organic solvents and soluble in three parts glasses of water. It’s made in vast quantities, mostly as a precursor to the industrial solvent methyl ethyl ketone. Because 2-butanol is chiral, it can be separated into two stereoisomers: (R)-()-2-butanol and (S)-(+)-2-butanol. It’s usually found as a racemic combination, which is a 1:1 mixing of the two stereoisomers.
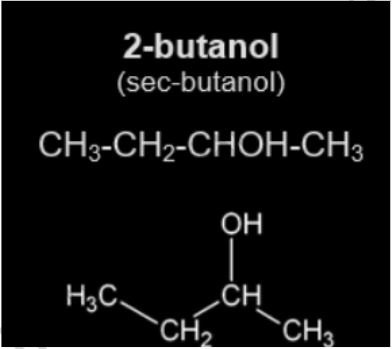
Although 2-butanol is employed as a solvent in some cases, it is primarily transformed to butanone (methyl ethyl ketone, MEK), a major industrial solvent found in many household cleaning products and paint removers. Due to health concerns and new restrictions, most paint removers have stopped utilizing MEK in their solutions. The volatile esters of 2-butanol have a pleasant fragrance and are used as perfumes or artificial flavours in tiny concentrations.
Significance of Identification of secondary alcohol in NEET exam
Understudies should have a solid view of the entire liable to complete the NEET evaluation. While each part is critical, and you should never dismiss any piece of your schedule, there is one average pack that you ought to truly zero in on. Since floating through the NEET evaluation is an especially enormous achievement in a student’s life, picking the best survey material is fundamental. The essential goal of Infinity Learning is to present confidence in our students. In this way, we composed the science responses to determine every request that a student would present. Since our responses are in pdf configuration, students can get to them at whatever point and from any area.
FAQs
Fermentation produces alcohol. The most common and useful alcohol, ethanol, is made by fermenting glucose (a sugar created by the hydrolysis of starch) in the presence of yeast. To make ethanol, the temperature must be kept below 37 degrees Celsius. Butanol can also be created using fermentation techniques. The addition of water to alkenes produces low-molecular-weight alcohols such as ethanol, isopropanol, 2-butanol, and tert-butanol. These alcohols are important in the industry.
As antibacterial rubbing alcohol, isopropyl alcohol is mixed with water. It's also found in aftershave lotions, hand lotions, and other beauty products. It's utilized in the manufacturing of cosmetics, pharmaceuticals, shellacs, and gums, as well as the denaturation of ethanol (ethyl alcohol).
Because isopropanol is ineffective against viruses that are not encapsulated, denatured ethanol is thought to be better as a virucidal disinfectant. What is the process of making alcohol?
What is the Purpose of Isopropyl Alcohol?
Is Denatured Alcohol an Antimicrobial?









