Table of Contents
A chemical reaction’s activation energy is proportional to its rate. In particular, the larger the activation energy, the slower the chemical reaction. The minimal amount of additional energy required by a reactive molecule to be turned into the product is defined as activation energy. Certain chemical bonds are destroyed and new ones are established during chemical reactions. The activation energy is the additional energy required for any reaction to occur. As a result, it is always positive.
Chemical processes require a certain amount of energy to begin. The activation energy is the minimal amount of energy necessary to initiate a reaction.
Svante Arrhenius, a Swedish chemist, discovered activation energy in 1889. To characterize the relationship between temperature and reaction rate, Arrhenius derived his distinctive equation. The Arrhenius equation is essential for estimating the rates of chemical processes and, more crucially, the amount of energy required to initiate them.
K is the reaction rate coefficient in the Arrhenius equation (the rate of reaction). T is the absolute temperature (typically measured in kelvins), A is the frequency factor (how frequently molecules collide), R is the universal gas constant), and Ea is the activation energy.
It is not essential to know the value of A to compute Ea because this may be determined by observing the fluctuation in reaction rate coefficients with temperature.
The minimal amount of additional energy required by a reactive molecule to be turned into the product is defined as activation energy. It is also defined as the smallest amount of energy required to activate or energize molecules or atoms in order for them to conduct a chemical reaction or transformation.
Ea represents activation energy. It is commonly expressed in joules (J), kilojoules per mole (kJ/mol), or kilocalories per mole (kcal/mol).
Factors Affecting Activation Energy
The activation energy is determined by two parameters:
-
The Nature of Reactants
Because there is an attraction between reacting species in the case of an ionic reactant, the value of (Ea) will be below. In the case of a covalent reactant, the value of Ea will be largely due to the energy necessary to break the older bonds.
-
Catalyst Effect
A positive catalyst creates an alternate path in which the value of Ea is low, whereas a negative catalyst creates another path in which the value of Ea is high.
Activation Energy and Rate of Reaction:
A chemical reaction’s activation energy is proportional to its rate. In particular, the larger the activation energy, the slower the chemical reaction. This is due to the fact that molecules can only finish the reaction after they have passed beyond the activation energy barrier. The greater the barrier, the fewer molecules will have the energy to cross at any one time.
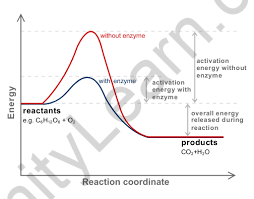
Activation Energy Graph:
Activation Energy Formula:
According to the Arrhenius equations, the rate of a chemical reaction is proportional to the magnitude of the activation energy:
The formula used to find the value of Activation Energy, Ea is;
K = Ae-Ea/RT
Where
A = Arrhenius Constant
K = Rate Constant
R = Gas constant = 8.34J/K/mol
Ea = Activation Energy
K = Ae-Ea/RT
Taking log on both sides
ln K = ln A – (Ea /RT)ln e
2.303 log K = 2.303 log A – Ea/RT
log K = log A – Ea/2.303RT
Activation Energy of A Reaction
The activation energy is equal to the difference between the reaction’s threshold energy and the average kinetic energy of all reacting molecules.
Each reaction has its own Ea value, which defines the proportion of total collisions that are effective. This indicates that if a reaction’s activation energy is low, many molecules have it, and the proportions of effective collisions are significant. The reaction is moving at a rapid rate. When the activation energy is high, the percentage of effective collisions is modest, and the reaction is sluggish.
Certain chemical bonds are destroyed and new ones are established during chemical reactions. When a glucose molecule is broken down, for example, bonds between the molecule’s carbon atoms are broken. Because they are energy-storing connections, when they are broken, they release energy. However, in order for the bonds to dissolve, the molecule must be twisted in some way. This deformed condition, known as the transition state, requires a little amount of energy to achieve: it is a high-energy, unstable state. As a result, reactant molecules do not stay in their transition state for long, but rather swiftly move to the following phases of the chemical reaction.
Forward Reactions: Reactions in which the product is derived from reactants.
Backward Reactions: These are reactions that occur when a reaction transitions from products to reactants.
Catalysts
A catalyst is a chemical substance that either speed up or slows down a chemical process. When it comes to the activation energy, a catalyst reduces it. The energy of the initial reactants, however, stays constant. A catalyst only modifies the activation energy.
Types of Catalyst
- Positive Catalysts
A positive catalyst is a catalyst that aids in increasing the pace of reaction or assisting the reaction to proceed swiftly. By accepting a narrower route, such a catalyst reduces activation energy and hence increases the pace of reaction.
- Negative Catalysts (Inhibitors)
Negative catalysts are catalysts that reduce, delay, or aid in the slowing of the rate of reaction. Because a negative catalyst increases activation energy by adopting a longer alternative path, this is the case.
Activated Complex
When reactant molecules contact at their most energetic position, an intermediate is created that remains in equilibrium with the primary reactant. If the energy of this intermediate complex is equal to or more than the Threshold Energy, it will be transformed into a product.
A+B ⇄ A——B→A-B
FAQ’s
What Function Does Activation Energy Serve?
During a chemical reaction, some bonds break, and others form. As a result, extra energy, i.e. energy beyond the average kinetic energy of the interacting molecules, is delivered to them so that they can move together, overcome forces of repulsion, and break bonds in order to convert. The activation energy is the energy that assists in this process.
Is it possible for Activation Energy to be Negative?
No, it does not. The activation energy is the additional energy required for any reaction to occur. As a result, it is always positive. When the activation energy is smaller at lower temperatures, the rate constant K approaches the pre-exponential factor.
What Is the Relationship Between Activation Energy and Temperature?
As the temperature rises, so does the kinetic energy of molecules. As the temperature rises, so does the collision between interacting molecules, and hence the activation energy.









