Table of Contents
Introduction
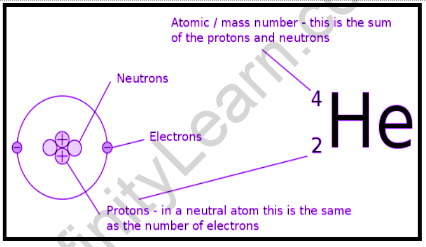
An atom can be thought of as a miniature solar system, with such a huge nucleus at the center and little electrons orbiting it. An atom’s bonding capacity is dictated by its electrons. Hydrogen atoms, for example, contain one electron in their natural state and will rapidly (and sometimes violently) bind with oxygen. The hydrogen bonding capacity was the cause of the airship Hindenburg’s 1937 disaster. Helium atoms, which have two electrons in their natural state and will not combine with oxygen, would have been a preferable choice for completing the Hindenburg. The number of atomic nuclei of each atom of an element is defined as its atomic number (Z). This indicates that the number of protons is the feature that differentiates each element from others.
| S.NO | CONTENT |
| 1 | INTRODUCTION |
| 2 | A BRIEF OUTLINE |
| 3 | IMPORTANT CONCEPTS |
| 4 | SIGNIFICANCE OF ATOMIC NUMBERS |
| 5 | FAQ’S |
A brief outline
The atomic number is the number of protons in a nucleus that always balances the number of electrons in orbit around that nucleus. As a result, all atoms with the same atomic number belong to the same element. Neutrons are also found in the nuclei of atoms, and they help to hold the nucleus together. A neutron is somewhat heavier than a proton and has no electrical charge. Since a neutron could decay into a proton plus an electron, it’s sometimes helpful to imagine a neutron as a mixture of an electron and a proton, though this is an oversimplification at best. A neutron has no impact on the number of electrons orbiting the nucleus as it has no charge. A neutron, on the other hand, is substantially heftier than a proton and can significantly increase the weight of an atom. The atomic weight is the total weight of an atom. It is roughly equal to the number of protons and neutrons, with the electrons adding a bit extra.
The majority of stable isotopes do not decay radioactively, although a handful does. The phrase “stable” refers to an isotope that decays radioactively relatively slowly. Bismuth-209 is an example. Bismuth-209 is a stable radioactive isotope that undergoes alpha decay. Tellurium-128 decays by beta decay. Let’s look at the few instances of isobars in action: 1) Uranium isobars can be used in nuclear reactors. 2) Iodine isobars are used to treat goiter. 3) Isobars of cobalt could be used to treat cancer.
Important concepts
Isotopes:
Atoms with the same number of protons yet different quantities of neutrons are called isotopes. Isotopes, in other terms, have distinct atomic weights. Different versions of a single element are known as isotopes. There are 250 isotopes for each of the 90 found natural elements, as well as more than 3,200 radioactive isotopes, some natural and some manmade. There are many isotope forms for every element on the periodic table. Isotopes of a single element have essentially identical chemical properties, with the exception of hydrogen isotopes, which have a considerable variation in the size of the hydrogen nucleus due to the number of neutrons.
Because isotopes’ physical qualities are typically dependent on mass, they differ from one another. The most abundant isotopes of natural elements, with the exception of hydrogen, have had the same number of protons and neutrons. Protium is the most prevalent hydrogen isotope, with one proton but no neutrons.
Isotope of an element has:
- Carbon 12 and 14 are two isotopes of carbon, including one with six neutrons and the other with eight (both with 6 protons). Carbon-12 seems to be a radioactive isotope, whilst carbon-14 is a stable isotope (radioisotope).
- Uranium-235 and uranium-238 would both be found natural elements in the Earth’s mantle. Both of them will have a short half. Uranium-234 is produced as a result of decaying.
Isobars:
Atoms with the same number of nucleons are referred to as isobars. Different chemical elements have different isobars with different atomic numbers but the same mass number. The word isobars were coined by Alfred Walter Stewart in 1918. It comes from the Greek term isos, which means “equal” and “bars,” which means “weight.”
Isobar of an element has:
Argon, potassium, and calcium, for example, all have atoms with the same mass number of 40. Isobars are 18Ar40, 19K40, and 20Ca40, where the subscripts 18, 19, and 20 represent the atomic numbers of the three elements, respectively. Because the atomic numbers differ, the chemical characteristics differ as well. Below are the structures of argon, potassium, and calcium.
Significance of atomic number in NEET exam
Experts at Infinity Learn can assist you in comprehending all vital ideas, and you can quickly get its free PDF online resource on all subjects to achieve excellent outcomes in NEET exams. Getting a second view on many issues is beneficial because a theory may appear simple but be quite complex to comprehend. In these situations, relying on infinite learning’s NCERT solutions as a reference is the best option. If properly understood, chemistry is the highest-scoring topic. The structure of atoms is the foundation of chemistry, and it is through this that you will study and comprehend the smallest unit of any substance. As a result, it is critical to comprehend the chapter and practice it thoroughly.
Also read: Important Topic Of Physics: Orbitals Shapes
FAQs
Why are isobars and isotopes present in elements?
Atomic Element has distinct isotopes due to the existence of a varied number of neutrons in atoms of the same element. Owing to the equivalent mass number (s) of neutrons and protons, various elements might just have varying isobars.
What are the benefits of isotopes?
The stable isotopes of an element have the same chemical behavior as the stable isotopes, while the unstable isotopes undergo spontaneous decay and emit radiation before reaching a stable state. Food preservation, archaeological object recognition, and medical testing and treatment all benefit from radioisotope activity.
What role does isobar chemistry play in chemistry?
Even if you're at the same altitude, atmospheric pressure varies depending on where you are. Isobars are reference lines that run alongside a path and have the same pressure all the way along. The user can receive a realistic approximation at any map point using a series of lines, each denoting a site where the pressures have a comparable set value. He can do so by reading the line's pressure if it passes through the interesting site precisely, or by interpolating based on the closest isobars, which are the ones with greater pressure than the intriguing place and the isobar with lower pressure.









