Table of Contents
The boiling point of a substance is the temperature at which the vapour pressure of a liquid equals the pressure surrounding the liquid, and the liquid converts to vapour. The boiling point of a liquid varies with the surrounding atmospheric pressure. In a partial vacuum, the boiling point of a liquid is lower than the boiling point of the same liquid at atmospheric pressure. A liquid’s boiling point at high pressure is higher than the identical liquid’s boiling point at atmospheric pressure. At sea level, water boils at 100 degrees Celsius (212 degrees Fahrenheit), while at 1,905 metres (6,250 feet) altitude, it boils at 93.4 degrees Celsius (200.1 degrees Fahrenheit). Different liquids will boil at different temperatures for a given pressure.
The normal boiling point of a liquid (also known as the atmospheric boiling point or the atmospheric pressure boiling point) is the special case in which the liquid’s vapor pressure in one atmosphere equals the stated atmospheric pressure at sea level. At that temperature, the liquid’s vapour pressure is high enough to overcome atmospheric pressure and allow vapour bubbles to form inside the liquid’s bulk. Since 1982, the International Union of Pure and Applied Chemistry (IUPAC) has defined the standard boiling point as the temperature at which boiling occurs at one bar of pressure.
Overview
Boiling point is defined as the temperature at which liquid vapour pressure equals atmospheric pressure. The boiling point of a liquid is defined as the temperature at which its saturated vapour pressure equals the surrounding atmospheric pressure. The boiling point of any material is the temperature at which the material transitions from the liquid phase to the gas phase. For water, this occurs at 100 degrees Celsius. The Celsius scale was designed around the ice/water melting point and the liquid water/vapor boiling point. The boiling point of each substance is different.
A substance’s boiling point is affected by the pressure in its surroundings. The atmospheric pressure in mountainous terrains (at high altitudes) is lower than the atmospheric pressure at sea level. This is why food in mountainous areas cooks at a slower rate (the lower atmospheric pressure causes water to boil at temperatures below 100C). As long as heat is applied to the surrounding system, the temperature begins to rise again once all of the particles in the liquid phase have been transformed into the gas phase. The particle’s kinetic energy increases as the temperature rises.
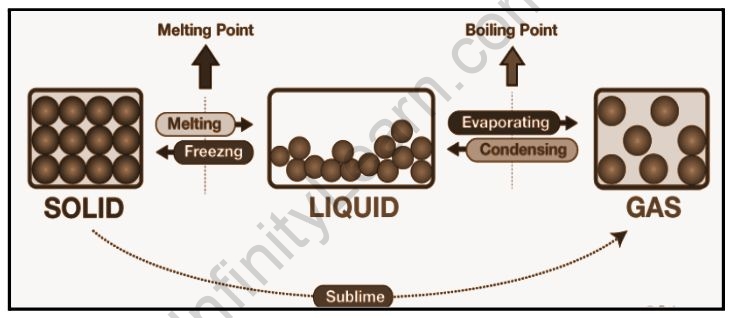
Melting and boiling points
The temperature at which a liquid’s vapour pressure equals the atmospheric pressure of the liquid’s surroundings is known as the boiling point. At this temperature, the liquid turns into a vapour. The surrounding pressure determines the boiling point of a liquid.
A liquid’s boiling point at high pressure is higher than its boiling point at atmospheric pressure. For a given pressure, the boiling points of various liquids differ.
The melting point is typically defined as the temperature at which a material transitions from a solid to a liquid. The melting point of a liquid is the temperature at which solid changes from solid to liquid at atmospheric pressure. This is the point where both the liquid and solid phases are in equilibrium. The substance’s melting point varies with pressure and is specified at standard pressure.
| Name of the substance | Boiling point(K) | Melting point(K) |
| Aluminium | 2740 | 932 |
| Copper | 1460 | 1359 |
| Gold | 2933 | 1336 |
| Hydrogen | 20.3 | 13.8 |
| Mercury | 630 | 234 |
Melting point
We refer to the temperature at which the solid and liquid forms of a pure substance can exist in equilibrium as the melting point. The temperature of a solid rises until it reaches the melting point when heated. More heat will then convert the solid to a liquid without changing the temperature. The additional heat will raise the temperature of the liquid once all of the solid has melted. The melting temperature of crystalline solids is a distinguishing figure used to identify pure compounds and elements. The majority of mixtures and amorphous solids melt at different temperatures.
The melting temperature of a solid is generally thought to be the same as the freezing point of the corresponding liquid; however, because a liquid can freeze in different crystal systems and impurities lower the freezing point, the actual freezing point may differ from the melting point.
It is referred to as the freezing point or crystallization point when the temperature of the reverse change from liquid to solid is considered. Because of a substance’s ability to supercool, the freezing point may appear to be lower than its true value. When determining a substance’s “characteristic freezing point,” the actual methodology is almost always “the principle of observing the disappearance rather than the formation of ice, that is, the melting point.”
Silver melting point
In thermodynamics, the melting point denotes the point at which a solid and a liquid can coexist in equilibrium. With no temperature change, adding heat will convert the solid to a liquid. The melting point of a substance is pressure-dependent and is typically specified at standard pressure. We refer to the temperature at which a liquid changes to a solid as the freezing point or crystallization point.
The melting point of Silver is 961.78°C
The boiling point of mercury
Mercury is a liquid metallic element that has a wide range of applications. It is also a hazardous element to the environment and the workplace, as well as corrosive to many materials. A knowledge of mercury solubility is useful in addressing problems that necessitate knowledge of metal concentrations in our surroundings’ liquids and vapours.
Mercury is a chemical element that is the only common metal that is liquid at room temperature. Mercury is a transition metal, one of the elements found on the periodic table between Groups 2 (IIA) and 13 (IIIA). Since the earth’s formation, the same amount has existed on the planet. Mercury, on the other hand, can cycle in the environment as a result of both natural and human activity. In addition, mercury metal has found numerous new applications in electrical devices and electrochemistry. Mercury metal is a highly flammable liquid with a measurable vapour pressure at room temperature.
Also read: Types of Bonding
FAQs
What is a substance's boiling point?
The boiling point of a pure substance is the temperature at which it changes from a liquid to a gaseous state. The vapour pressure of the liquid is equal to the applied pressure at this point.
Is it dangerous to touch Mercury?
Mercury is a highly toxic or poisonous substance to which people can be exposed in a variety of ways. If swallowed, such as from a broken thermometer, it will primarily pass through your body, absorbing very little. If you touch it, a small amount may pass through your skin, but not enough to cause harm.








