Table of Contents
Chemistry is concerned with the process by which subatomic particles bond together to form atoms. It is also concerned with how atoms bond together to form molecules. Electrons surround the atomic nucleus in regions known as orbitals in the atomic structure. Evey orbital shell can only hold so many electrons. When the nearest orbital shell is full, new electrons begin to accumulate in the next orbital shell out from the nucleus, and this process continues until that shell is also full. As larger atoms have more electrons than smaller atoms, the collection of electrons continues in ever-widening orbital shells. Once two atoms combine to form a molecule, their electrons combine by mixing into openings in each other’s orbital shells. The establishment of bonds by the molecule, like the collection of electrons by the atom, begins at the nearest available orbital shell opening and expands outward.
The information about bond parameters from various chemistry-related articles is available here. Bond parameters are an important topic in chemistry. Students who want to flourish in chemistry need to be well known about this to get deep knowledge about it to do well on their exams. The definitions and brief explanations are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional chemistry help.
Overview
We can say that the chemical bond is a long-lasting attraction between atoms, ions, or molecules that allows chemical compounds to form. The ionic bonds are established by the electrostatic force of attraction between oppositely charged ions, whereas covalent bonds are formed by the sharing of electrons. The strength of chemical bonds varies a lot.
We have seen different types of bonds, including ionic bonds and covalent bonds. In this, covalent bonds can be described using a variety of bond parameters such as bond length, bond angle, bond order, and bond energy (also known as bond enthalpy). These bond parameters reveal information about a chemical compound’s stability as well as the strength of the chemical bonds that hold its atoms together. Numerous atoms must join together to become stable. Such a combination happens as a result of bond formation. As a result, each bond is associated with a characteristic.
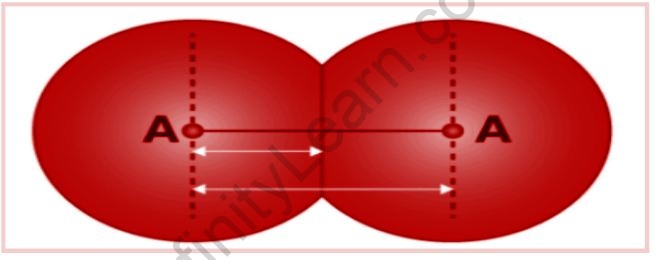
Bond Parameters Definition
Bond parameters are the parameters used to characterize covalent bonds, such as bond length, bond angle, and bond enthalpy. That is, bond parameters are a collection of various features or characteristics that can be observed in bonds.
Bond Length
The bond length is described as the difference between the nuclei of two bonded atoms in equilibrium. The shorter the bond, the stronger the force of attraction between the bonding atoms. The length of the bond, on the other hand, increases with the size of the atom.
It really is measured using spectroscopy, X-ray diffraction, and electron diffraction. The bond length is determined by each atom in the bonded pair. In the case of a covalent bond, each atom’s contribution is its covalent radius.
The bond length is determined by the following factors:
- Bond Multiplicity: Bond length decreases as bond multiplicity increases.
- Size of an Atom: The length of a bond is directly proportional to the size of an atom. The bond length grows in proportion to the size of the atoms.
The shorter the bond, the stronger the attraction between the bonding atoms. However, the length of a bond is proportional to the size of the atom. It should also be noted that in the case of a covalent bond, the contribution of each atom is referred to as the atom’s covalent radius.
Tied or bonded atoms absorb thermal energy from their surroundings and constantly vibrate. Such a vibration also causes the bond length to vary. As a result, it is critical to remember that the bond length of a covalent bond describes the average distance between the nuclei of the atoms involved.
Bond lengths are indeed directly proportionate to the atomic radii of the atoms involved. The periodic trends observed in element bond lengths are the same as the periodic trends observed in element atomic radii.
Bond Angle
Bond angle is the angle formed by two bonds, i.e., the angle formed by two orbitals in a complex molecule or an ion that contains a pair of bonding electrons around the central atom. Such an angle is typically measured in degrees and calculated further using the spectroscopic method.
This provides a clear picture of how bonded electron pairs are distributed around the atoms and aids in determining the shape of the molecules. This also provides an insight into how bonded electron pairs are distributed around the atoms and how the shape of the molecules is determined.
Bond Enthalpy
The quantity of energy required to break one mole of a specific type of bond between two atoms in a gaseous state is referred to as the Bond Enthalpies. Bond enthalpy is proportional to the strength of the molecule-to-molecule bond.
In the particular instance of polyatomic molecules, the bond enthalpy of two bonds of the same type can differ. For example, the bond enthalpy of two O-H bonds in a water molecule differs. Because of differences in bond enthalpy, polyatomic molecules have average bond enthalpy.
Also, it is worth noting that the bond energy is not the same as the bond dissociation energy. It is the latter enthalpy change associated with a bond’s homolytic cleavage, whereas the former is the average of the bond dissociation enthalpies of all total bonds (of a specific type) present in a molecule.
Bond Order
The bond order is the number of bonds that form between two atoms in a molecule, according to the Lewis description of covalent bonds. The bond order of isoelectronic molecules or ions is the same.
For example, the two isoelectronic molecules, F2 and O22-, have the same bond order of 1. The larger the bond’s order, the greater the bond’s enthalpy and the shorter the bond’s length.
A bond order in H2 is one when one electron pair is shared, two in O2 when two electron pairs are shared, and three in N2 when three electron pairs are shared.
As per the molecular orbital theory, the bond order of a covalent bond is equal to half of the difference between the bonding and antibonding electron counts, which can be symbolized using the formula:
Bond Order =(1/2)* (total number of bonding electrons – total number of antibonding electrons)
Also read: Important Topic of Chemistry: Covalent Bond
Frequently Asked Questions
Which bond is the strongest bond?
We discover that triple bonds between the same two atoms are stronger and shorter than double bonds and that double bonds between the same two atoms are stronger and shorter than single bonds.
What is the enthalpy of bond dissociation?
Bond enthalpy, also known as bond-dissociation enthalpy, average bond energy, or bond strength, is the amount of energy contained in a bond between atoms in a molecule. In the real sense, it is the energy that must be applied to the homolytic or symmetrical cleavage of a bond in the gas phase.
What is meant by bond angle?
A bond angle is an angle formed by three atoms over at least two bonds. An angle of torsion for four atoms bonded together in a chain is the angle formed by the first three atoms and the plane formed by the last three atoms.









