Table of Contents
In general, buffers are solutions with a stable concentration of hydrogen ions and thus typically no change in pH that is almost independent of dilution and changes very little with small additions of a strong acid or alkali. Most of the chemical reactions are influenced by the acidity of the solution in which they occur. For a given reaction to occur or occur at a suitable rate, the pH of the reaction medium must be controlled. This control is provided by buffer solutions, which are solutions that keep a specific pH constant. pH is especially important in biochemical reactions. Most biological molecules contain groups of atoms that can be charged or neutral based on pH, and whether these groups are charged or neutral affects the molecule’s biological activity significantly. In every multicellular organism, the fluid within the cell and the fluids surrounding the cells have a distinct and nearly constant pH. This pH is maintained in a variety of ways, the most important of which is through buffer systems.
The information about buffer solutions from various chemistry-related articles is available here. In general, buffers are solutions with a stable concentration of hydrogen ions and thus typically no change in pH that is almost independent of dilution and changes very little with small additions of a strong acid or alkali. Students who want to flourish in chemistry need to be well known about buffer solutions to get deep knowledge about it to do well on their exams. General concepts on buffer solutions and their types are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional chemistry help.
Overview
Whenever a few drops of a strong acid or base are added to water, the concentration of hydrogen ions changes dramatically. Many industrial, chemical, and biological processes require a solution whose pH does not change significantly when small amounts of strong acids and strong bases are added. Many fluids, including blood, have specific pH values, and variations in these values indicate a malfunctioning body. Controlling pH is also important in a variety of chemical and biological processes. A specific pH is required for the manufacture and use of many medical and cosmetic products.
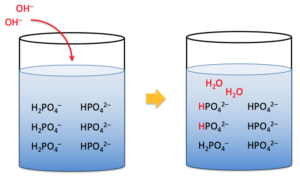
A buffer solution (also known as a pH buffer or hydrogen ion buffer) is an aqueous solution made up of a weak acid and its conjugate base, or vice versa. Whenever a small amount of strong acid or base is added to it, the pH changes very little. We can say that, buffer solutions are used in a wide range of chemical applications to maintain a nearly constant pH. There are many systems in nature that use buffering to regulate pH. The bicarbonate buffering system, for example, is used to regulate blood pH, and bicarbonate also acts as a buffer in the ocean.
The pH of buffer solutions changes very little when a very small amount of strong acid or strong base is added. As a result, they are used to keep the pH constant. Solutions of something like a weak acid and its conjugate base, or a weak base and its conjugate acid, can maintain pH and serve as buffers.
Buffer solutions
Buffers are solutions with a stable hydrogen ion concentration and, as a result, usually no change in pH that is almost independent of dilution and changes very little with small additions of a strong acid or alkali. In simpler terms, a buffer solution or simply a buffer is a solution that resists any change in pH when a small amount of a strong acid or a strong base is added to it. As a result, buffers have acidity as well as reserve alkalinity.
Specific solutions, such as ammonium acetate, tend to resist any change in hydronium ion concentration or pH when a small amount of a strong acid or a strong base is added to them. This is referred to as a solution’s buffer action.
There will be two ways to prepare a buffer solution with a specific pH. In the first method, prepare a solution with an acid and its conjugate base by dissolving the buffer’s acid form in approximately 60% of the volume of water required to obtain the final solution volume and the pH of the solution should then be measured using a pH probe. In fact, a strong base, such as NaOH, can be used to raise the pH to the desired level. If the buffer is composed of a base and its conjugate acid, the pH can be adjusted with a strong acid such as HCl. When the pH is correct, dilute the solution to the final volume desired.
Conversely, you can prepare both the acid and base forms of the solution. The concentration of the buffer in both solutions must be the same as the concentration of the buffer in the final solution. Add one solution to the other while monitoring the pH to get the final buffer.
The Henderson–Hasselbalch equation arithmetically connects a solution’s measurable pH with the acid’s pKa (which is equal to -log Ka). The equation can also be used to calculate the pH of a buffer solution and the equilibrium pH of an acid-base reaction. The formula for pKa for a weak acid or buffer can be used to derive the equation.
Types of buffer solution
The two types of buffer solutions that are extensively classified are acidic and basic buffers.
Acidic buffer solution: Acidic buffer solutions contain equimolar amounts of a weak acid and its salt, as well as a strong base. These solutions are used to maintain the acidity of the environment. To create an acidic pH, an acid buffer is made by combining a weak acid and its salt with a strong base. An aqueous solution containing equal parts acetic acid and sodium acetate has a pH of 4.74. Furthermore, these liquids have a pH of less than seven. Such solutions contain a weak acid and its salt.
Basic buffer solution: A weak base and its salt are equimolar with a strong acid in a basic buffer solution. Such buffer solutions are used to maintain basic conditions. A weak base and its salt are combined with a strong acid to produce a basic buffer with a basic pH. The pH of an aqueous solution of equal parts ammonium hydroxide and ammonium chloride is 9.25. The pH of these solutions is greater than seven. They are made up of a weak base and a weak base salt.
FAQs
Why are Buffer Solutions Important?
The buffer is any solution that can withstand changes in pH when an acidic or basic component is added. It can neutralise small amounts of added acid or base, allowing the solution to maintain a fairly constant pH. This is critical for processes and/or reactions that require distinct and stable pH ranges.
What are types of buffer solutions?
Buffer solutions are broadly classified into two types: acidic buffer solutions and alkaline buffer solutions. Acidic buffers are solutions with a pH lower than 7 that contain a weak acid and one of its salts.
What is an unbuffered solution?
Because the added hydronium or hydroxide ions have nothing to react to within the unbuffered solution, their concentrations rise rapidly, causing the pH to rise significantly.







