Table of Contents
Coagulation flocculation in water treatment involves the addition of compounds that promote the clumping of fines into larger flocs, allowing them to be more easily separated from the water. Coagulation is a chemical process that involves charge neutralisation, whereas flocculation is a physical process that does not involve charge neutralisation. Coagulation-flocculation can be used as a preparatory or intermediate step between other water or wastewater treatment processes such as filtration and sedimentation. Although iron and aluminium salts are the most commonly used coagulants, salts of other metals such as titanium and zirconium have also been found to be highly effective. The type of coagulant used, its dose and mass; the pH and initial turbidity of the water being treated; and the properties of the pollutants present all have an impact on coagulation. Pretreatments such as oxidation also have an impact on the coagulation process’s effectiveness. Because colloidal particles have surface electrical charges that repel each other, particles settle very slowly or not at all in a colloidal suspension. This surface charge is commonly measured in terms of zeta potential, which is the electrical potential at the slipping plane.A coagulant with the opposite charge is added to the water to overcome the repulsive charge and “destabilise” the suspension, causing it to coagulate. Negatively charged colloidal particles, for example, are coagulated with alum to form positively charged ions. Once the repulsive charges are neutralised (opposing charges attract), van der Waals’s force causes the particles to cling together (agglomerate) and form micro floccules.
Overview
Suspended particles cannot be completely removed by settling alone. Large, heavy particles settle out quickly, but smaller and lighter particles settle out slowly or not at all. As a result, the sedimentation step is usually preceded by coagulation, a chemical process. Chemicals (coagulants) are added to the water to coagulate the nonsettling particles into larger, heavier masses of solids known as floc. The most common coagulant used for water purification is aluminium sulphate (alum). Other chemicals, such as ferric sulphate or sodium aluminate, could be used as well. Coagulation is typically performed in two stages: rapid mixing and slow mixing. Rapid mixing ensures that the coagulants are evenly distributed throughout the water and that the chemical reaction is complete. This is sometimes achieved by adding the chemicals just before the pumps and allowing the pump impellers to do the mixing. A small flash-mix tank, on the other hand, usually provides about one minute of detention time. Coagulation can be defined in chemistry as one of the many properties exhibited by colloidal solutions. A colloid is a heterogeneous mixture of one single substance containing very fine particles (a dispersed phase) dispersed in another substance (a dispersion medium). Few substances, such as metals and their sulphides, cannot be simply mixed with the dispersion medium to form a colloidal solution. Their colloidal solutions are created using some unique techniques. This type of solution is known as a lyophobic solution. This type of colloidal solution always has some charge on it. The presence of charge on colloidal solutions indicates their stability.If we can remove the charge on the solution, the particles will get closer to each other and accumulate to form precipitate and aggregates under gravity action. Precipitation or coagulation refers to the process of particle accumulation and settling down.
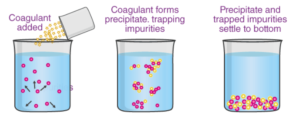
Coagulation meaning
Coagulation is the process by which colloidal particles aggregate or accumulate in order to settle as a precipitate. Metals, their sulphides, and other substances cannot be simply mixed with the dispersion medium to form a colloidal solution. Their colloidal solutions are created using some unique techniques. Such sols are referred to as lyophobic sols.
These colloidal solutions are always charged in some way. The charge on colloidal sols determines their stability. If we can remove the charge on the sol, the particles get closer to each other and accumulate to form aggregates, which precipitate under the influence of gravity. This process of particle accumulation and settling down is also known as coagulation or precipitation.
Blood coagulation
Blood coagulation or clotting is an important phenomenon that helps to prevent excessive blood loss in the event of an injury or trauma. When a wound is injured, the flow of blood stops. A network of fibrin threads forms the blood clot or coagulum. Deformed and dead formed elements (erythrocytes, leukocytes, and platelets) become trapped in this network The thrombin enzyme converts fibrinogen in the plasma to fibrin. It is a series of enzyme-catalyzed reactions in a cascade process. Plasma contains fibrinogen and other inactive blood clotting factors. An injury causes platelets or thrombocytes to release a variety of factors that start the blood clotting cascade. Calcium ions are essential in blood coagulation.
Blood coagulation results in haemostasis, or the prevention of bleeding or haemorrhage. Blood clotting begins with platelet activation and aggregation at exposed endothelial cells, followed by the deposition and stabilisation of cross-linked fibrin mesh. Primary haemostasis is caused by platelet aggregation and the formation of a plug at the site of injury, whereas secondary haemostasis is caused by the formation of a network of fibrin threads, which strengthens and stabilises the platelet plug. Secondary haemostasis is caused by two coagulation pathways: the intrinsic and extrinsic pathways. Both pathways converge at a point, resulting in fibrin activation and the formation of the fibrin network.
FAQ’s
How Do Coagulation Tests Work?
Coagulation tests are frequently performed in the same manner as any other major blood test. We may be instructed to stop taking certain medications prior to the test. There is no other preparation required. The doctor will sterilise a spot on the back of our hand or inside our elbow. They will then insert a needle into a vein. A minor stick is felt by the majority of people.
What Is the Definition of Coagulation?
Coagulation is a chemical process that causes the destabilisation of non-settleable particles. With the help of a coagulant, these particles form clumps. Colloidal particles and very fine solid suspensions in wastewater combine to form larger agglomerates that can be separated using various separation methods.
What causes colloidal solution coagulation?
The charge on colloidal sols determines their stability. If we remove the charge on the sol, the particles get closer to each other and accumulate to form aggregates, which precipitate under gravity. As a result, the electrolyte is added to the sol to neutralise the charge and settle particles that cause coagulation or precipitation.







