Table of Contents
Any of a class of chemical compounds with the molecular formula diazonium. R is an atomic grouping formed by removing a hydrogen atom from the structural formula of an organic compound. Diazonium salts are formed by reacting primary amines with nitrous acid (diazotization); their most distinguishing feature is their instability. Aliphatic diazonium salts exist only as brief intermediates, quickly dissolving into a nitrogen molecule and a carbonium ion; certain aromatic diazonium salts are stable enough to isolate, but readily react with nitrogen loss or the formation of azo compounds. Diazonium salts were first synthesised in 1858 from aromatic amines, and the dye industry quickly recognised their utility in the production of azo compounds. Colors from the visible spectrum are added to dyes suitable for a variety of fibres by modifying the chemical structures of the diazotized amines (the diazo components) and the compounds with which they react (the coupling components). The diazonium group can be replaced by a variety of atoms or groups of atoms, often with the assistance of copper or a copper salt; these reactions allow for the preparation of a wide range of aromatic derivatives. Hydrazine derivatives are formed through the chemical reduction of aromatic diazonium salts.Diazonium compounds, also known as diazonium salts, are a class of organic compounds that share the functional group R−N+2X− , where R can be any organic group, such as an alkyl or an aryl, and X can be an inorganic or organic anion, such as a halogen.
For instance, Halogen, Chlorine, Bromine, and so on. Diazonium compounds are another name for diazonium salts. We can make phenols by heating it in water. Furthermore, azo compounds can be synthesised by reacting diazonium salts with other aromatic compounds.
Overview
Diazonium salts are organic compounds with the formula R–N2+X–, where R can be any alkyl or aryl group and X can be halogens, hydrogen sulphate, or other organic compounds.
Aryl diazonium salts are frequently used as chemical intermediates. The diazonium group is easily replaced by a variety of functional groups, including –I, –OH, –F, –CN, and –H, which cannot be substituted directly into the aromatic ring. Furthermore, replacement patterns that are diametrically opposed to the norm can be generated (i.e., preparation of 1,3-dihalo substituted benzenes). The diazonium salt is not separated in the majority of these replacements.
Arenediazonium salts are a type of chemical that contains arene diazonium. The word di refers to two, aza to nitrogen, and onium to the ionic nature of the compound in the phrase “Diazonium salts.” As a result, diazonium salts are ionic compounds that contain N≡N. Diazonium salts are organic compounds that have triple bonds between Nitrogen atoms and either an alkyl or an aryl (benzene ring) on the other side. Diazonium salts are the transitional phase between azo dyes (or compounds are known to be popular colouring agents). Salts are named after the double nitrogen (diazo) found in ionic salts, where chloride molecules replace the nitrogen atom.
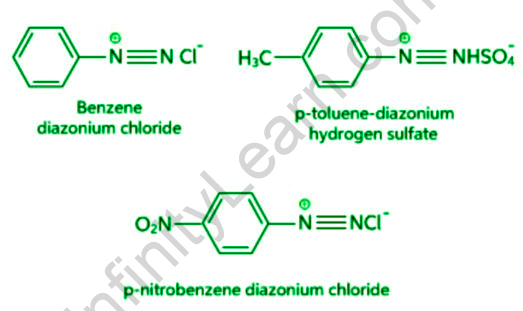
Diazonium Salts’ Properties
- They have an ionic nature.
- They are soluble in water.
- The salts of aryl diazonium are colourless crystalline solids.
- Although benzenediazonium chloride is soluble in water, it only reacts with it when it is warmed.
- Water does not dissolve benzenediazonium fluoroborate.
- At room temperature, it is fairly stable.
Diazonium salt reactions
Once synthesised, diazonium salts can be used as building blocks in a variety of other organic reactions. Let’s look at some of the more common reactions that our salts can undergo.
Reaction of Sandmeyer
Aromatic diazonium salts can be reacted with copper (I) chloride to form aryl chloride in a reaction known as the Sandmeyer reaction, after its discoverer Traugott Sandmeyer in the late 1800s. The diazonium salt loses a nitrogen molecule, and the diazo group on the aromatic ring is eventually replaced by a chlorine atom supplied by the copper (I) chloride reagent.

Synthesis of Phenols
When a diazonium salt is heated in water, the diazo component of the compound is replaced by a hydroxyl group (-OH), yielding a phenol. Phenols are important organic building blocks in pharmaceuticals and drug discovery projects.
Formation of diazonium salt
A diazonium salt is formed when amine reacts with nitrosonium ion. Because diazonium salt is usually unstable, it is usually made in situ. When the alkyl group is replaced with an aryl group, the salt is stable at 0 degrees Celsius and reacts with a variety of nucleophiles. Diazotization or dissociation is the process of converting an organic compound, typically primary aromatic amines, into diazonium salts. The diazonium group has been shown to be highly unstable under normal conditions, so it is rarely stored and is usually used immediately after preparation. The reaction of nitrous acid with aromatic amines is one of the most common methods of producing diazonium salt. When aniline (aromatic amine) reacts with nitrous acid, the diazonium salt benzene diazonium chloride is formed.
Because nitrous acid is a highly toxic gas, it is usually produced during the reaction (in situ) by reacting NaNO2 with a mineral acid. Temperature is another important parameter that governs product formation during the preparation of these salts; most diazonium salts are stable below 5o C. As a result, it is critical to keep the reaction temperature below 5oC, or else the diazonium group will decompose to give N2 as soon as it is formed.
Diazonium salts are formed in the presence of hydrochloric acid by combining an alkyl or aryl primary amine with sodium nitrite. The following are some examples of reactions involving diazonium salts: Sandmeyer reaction: the formation of an aryl chloride via the action of copper (I) chloride.
Formation of benzene diazonium chloride
Because it may be used to generate a number of different compounds, such as halobenzenes, benzenediazonium chloride is an important benzene-containing chemical. To make benzenediazonium chloride, first combine benzene and nitric acid in the presence of sulfuric acid, which produces nitrobenzene. The benzenediazonium chloride molecule is formed by combining nitrobenzene with nitrous acid in the presence of hydrochloric acid. The reaction mechanism for the formation of benzenediazonium chloride is somewhat complicated and consists of several steps. Aniline is used to make benzene diazonium chloride. When aniline reacts with nitrous acid at low temperatures (0-5 C), the product is benzenediazonium chloride. When the temperature rises, benzene diazonium chloride decomposes into phenol. As a result, when preparing benzene diazonium chloride, you must exercise caution with regard to temperature. To make benzenediazonium chloride, first combine benzene and nitric acid in the presence of sulfuric acid, which produces nitrobenzene. The benzenediazonium chloride molecule is formed by combining nitrobenzene with nitrous acid in the presence of hydrochloric acid.
FAQs
What exactly is diazonium salt?
Diazonium salts are a class of organic compounds that all have the same R-NH2+X– function group. In R-NH2+X–, R is an organic group. For instance, the alkyl or aryl groups. Furthermore, azo compounds can be formed by diazonium salts reacting with other aromatic compounds.
What exactly is the significance of diazonium salt?
Diazonium compounds are common reagents used in the synthesis of organic compounds, particularly aryl derivatives. Diazonium salts are light-sensitive and degrade when exposed to Ultraviolet or Violet light. This wealth contributed to their use in paper copying. A diazonium salt is used to paint paper or film for this process.
Why is it critical to keep diazonium ion cool?
We must maintain a low temperature throughout the diazotization and coupling reactions because at high temperatures, diazonium salts are produced by certain materials and give phenol by reacting at high temperatures with water, which will result in a major error in the experiments.









