Table of Contents
The tendency of an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond is denoted by the symbol. Both the atomic number and the distance between the valence electrons and the charged nucleus influence an atom’s electronegativity. The higher the associated electronegativity, the more electrons an atom or substituent group attracts. Electronegativity is a simple way to estimate the bond energy as well as the sign and magnitude of a bond’s chemical polarity, which characterizes a bond along a continuous scale from covalent to ionic bonding. The opposite of electronegativity is electropositivity, which describes an element’s proclivity to donate valence electrons. At the most basic level, electronegativity is determined by factors such as nuclear charge (the more protons an atom has, the more “pull” it will have on electrons) and the number and location of other electrons in the atomic shells (the more electrons an atom has, the farther from the nucleus the valence electrons will be, and as a result, the less positive charge they will experience—both because of their increased distance from the nucleus and because of the other electrons. The electronegativity scale, developed by Linus Pauling in 1932, is a widely used measure of the electronegativities of chemical elements. It lists the elements in decreasing order of electronegativity, with fluorine being the most electronegative and cesium being the least. The scale was created by comparing the energies associated with chemical bonds formed by various atom combinations. Measurements of atomic ionization potentials and electron affinities yielded a scale very similar to Pauling’s values.
Overview
Electronegativity is a property of an atom that increases with its attraction to the electrons of a bond. In a covalent bond, two bonded atoms with the same electronegativity values share electrons equally. Typically, electrons in a chemical bond are more attracted to one atom (the more electronegative one) than the other. A polar covalent bond is formed as a result. If the electronegativity values are extremely dissimilar, the electrons are not shared at all. An ionic bond is formed when one atom takes the bond electrons from the other atom.
The ability of an atom in a chemical bond to attract electrons to itself is known as electronegativity. Fluorine is the most electronegative element in the periodic table. Francium is the least electronegative or most electropositive element. The greater the difference in electronegativity values between atoms, the more polar the chemical bond formed between them.
Electronegativity is a property of an atom within a molecule, not a property of an atom in and of itself. As a result, electronegativity varies depending on an atom’s environment. However, most of the time, an atom behaves similarly in different situations. The nuclear charge, as well as the number and location of electrons in an atom, are all factors that influence electronegativity.
Because it is only a tendency, it is a dimensionless property. It essentially denotes the net result of atoms in various elements’ proclivity to attract bond-forming electron pairs. Electronegativity is measured on several scales. The most extensively used scale was designed by Linus Pauling. Fluorine is the most electronegative element on this scale, with a value of 4.0, and cesium is the least electronegative element, with a value of 0.7.
Electronegativity definition
Electronegativity is a chemical property that describes an atom’s or functional group’s proclivity to attract electrons. An atom’s electronegativity is affected by both its atomic number and the distance between its valence electrons and the charged nuclei. Electronegativity is a measure of an atom’s proclivity to attract a bonding pair of electrons. The Pauling scale is the most widely used scale. Fluorine (the most electronegative element) has a value of 4.0, and the least electronegative elements, cesium and francium have values of 0.7.
Most electronegative element
On the periodic table, fluorine is the most electronegative element. It has an electronegativity of 3.98. Fluorine is the smallest halogen, so it is the smallest element in the periodic table to attract electrons, making it the most electronegative element. To achieve stability, a fluorine atom requires only one electron to fill its outer electron shell. As a result, free fluorine exists as the F– ion. Fluorine has 7 electrons in its valence shell. As a result, if we add one more electron, the electrons in the valence shell will repel that incoming electron due to their smaller size and high electron density.
Electronegativity can be measured on a variety of scales. Linus Pauling created the most widely used scale.
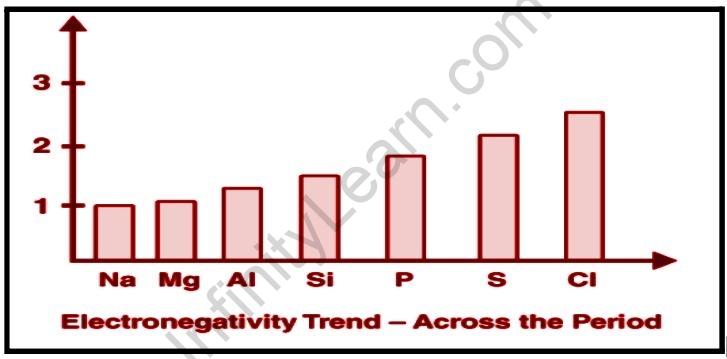
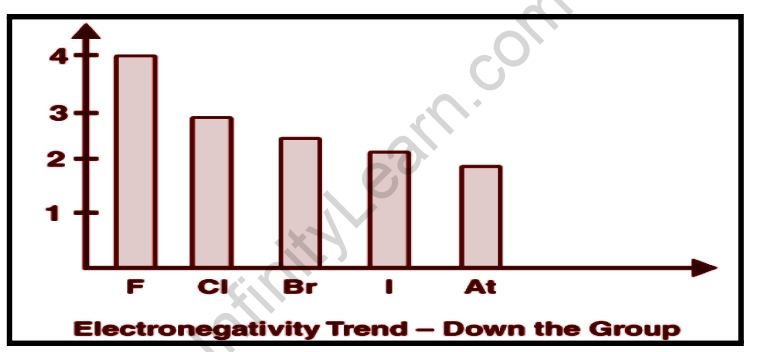
Size of an Atom Nuclear Charge Effect of Substituents Factors Affecting Electronegativity.
Electronegativity periodic table
Electronegativity is a chemical property that describes an atom’s ability to attract electrons to itself within a molecule. There is a significant difference in electronegativity between atoms on the left and right sides of the periodic table. Electronegativity is an important quantity in determining the nature of elements’ bonds and will be regarded as the most important factor in chemical bonding.
The periodic table of elements is shown below, along with the electronegativity table.
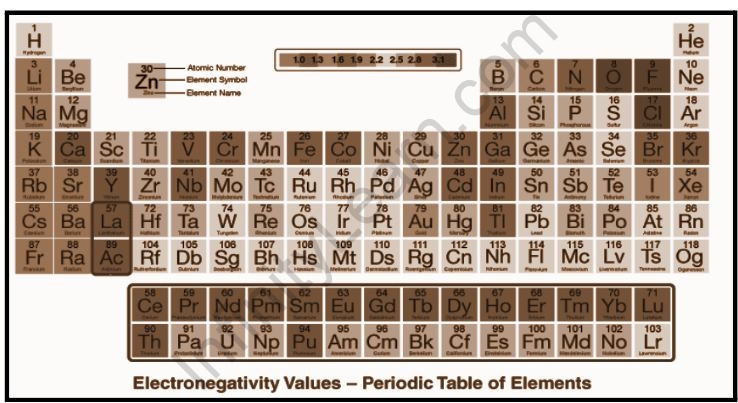
The article can help students understand the trends in electronegativity in periodic tables. Electronegativity is the focal point of this discussion. Electronegativity is an important property of an element or atom because it reveals information about an element’s or atom’s binding nature.
Also read: Important Topic of Chemistry: Lewis Structure
FAQs
Which definition of electronegativity is the most accurate?
Electronegativity is a measure of an atom's ability to attract a pair of electrons. The Pauling scale is the most commonly used. Fluorine is assigned a value of 4.0, and the least electronegative values, 0.7, are assigned to cesium and francium.
What exactly is high electronegativity?
Electronegativity decreases from top to bottom and increases from left to the right over time. Fluorine is thus the most electronegative element, while francium is one of the least electronegative elements.
What is the difference in electronegativity?
Electronegativity describes the degree to which an atom attracts electrons in a chemical bond. If the difference in electronegativity is greater than 1.7, the bond has an ionic character. If the difference in electronegativity is between 0.4 and 1.7, the bond has a polar covalent character.







