Table of Contents
Isomers are molecules with much the same chemical formula but just a differing atomic layout in space. This rules out whatever unusual arrangements caused by the molecule spinning as a whole or spinning around specific bonds. Structural isomerism occurs when the atoms that make up the distinct isomers are connected in a different order. Structural isomerism is a type of stereoisomerism that is discussed on its own page. Stereoisomerism occurs when the atoms that make up an isomer are linked in the same sequence but have a distinct spatial arrangement. Stereoisomerism can take many forms, one being geometric isomerism.
A brief outline
Geometrical isomerism definition
Cis–trans isomerism, also termed geometric isomerism or conformational isomerism, is a chemical word that refers to how atoms are arranged within molecules. “Cis” and “trans” are Latin prefixes that mean “this side of” and “the other side of,” respectively. In chemistry, cis denotes that now the functional groups (substituents) are on the same side of a plane, whereas trans denotes that they are on opposite (transverse) sides of the plane. Cis–trans isomers are stereoisomers, which are pairs of molecules with the same formula but functional groups oriented differently in three dimensions.
Both organic compounds and inorganic coordination complexes contain cis and trans isomers. For scenarios of conformation isomerism in which the two geometric forms readily interconvert, just like most open-chain single-bonded structures, the terms “syn” and “anti” are used instead of “cis” and “trans.” IUPAC considers “geometric isomerism” to be an outdated synonym for “cis-trans isomerism.”
Important concepts
Geometric Isomerism Examples
This section includes some cis-trans isomer examples as well as illustrations.
Organic compounds
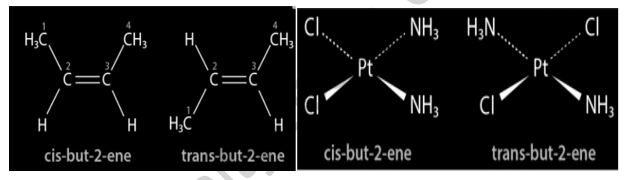
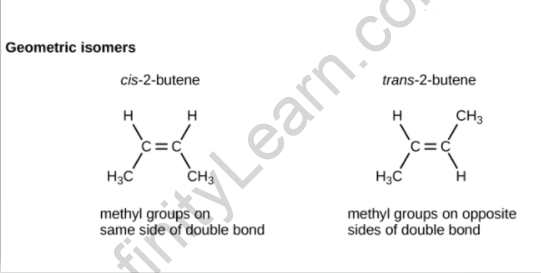
The existence of a double or triple bond within a molecule prevents bond rotation, resulting in cis-trans isomerism. The chemical molecule but-2-ene exhibits this form of isomerism. The cis and trans isomers of but-2-ene have now been depicted in the diagram below.
Inorganic compounds
Cis-trans isomers are known to exist in many diazenes and diphosphenes. Based on the location of the ligands, coordination complexes with square planar or octahedral geometries also exhibit cis-trans isomerism. Below is a diagram of the isomers of the coordination complex Pt (NH3)2Cl2. Similar ligands are found near one other in the cis isomer, but on opposing sides in the equivalent trans isomer.
Difference in properties
The physical properties of the cis-trans isomers of a molecule differ in many circumstances. Changes in the dipole moment of the molecule or variances in the spatial arrangement of atoms might cause these differences. Some of these distinctions are highlighted in the table below.
- The cis isomer of pent-2-ene does have a boiling point of 37°C, while the trans isomer has a boiling temperature of 36°C. Because the bond polarity is low, the change is minor.
- The cis isomer’s boiling point is 60.3ͦ C, while the trans-isomers is 47.5ͦ C, due to the polar nature of the bonds in 1,2-dichloroethylene (the C-Cl dipole moments in the trans isomer cancel each other out).
- Because of the differences in their characteristics, the cis and trans isomers of butanediol acid have extremely distinct reactivities. The cis isomer of maleic acid is maleic acid, while the trans isomer is fumaric acid.
- The cis-trans isomers of elaidic acid and oleic acid are elaidic acid and oleic acid, respectively. At room temperature, the former is solid (melting point = 43oC), whereas the latter is liquid (melting point = 13.4oC).
The physical properties of cis and trans isomers are frequently different. Variances in the geometry of the molecule or the total dipole moment cause differences between isomers in general. The boiling point of straight-chain alkenes, such as pent-2-ene, is 37 °C in the cis isomer and 36 °C in the trans isomer, although these variances can be quite slight. When polar linkages are present, such as in 1,2-dichloroethenes, the differences among cis and trans isomers can be significant. In this situation, the cis isomer has a boiling point of 60.3 degrees Celsius, while the trans isomer has a boiling temperature of 47.5 degrees Celsius.
Stability
Trans isomers are usually more stable than cis isomers in acyclic systems. This is owing to the cis isomer’s greater unfavourable steric interaction with the substituents. In a conclusion, trans isomers have lower exothermic heat of combustion, suggesting that they have been more thermochemically durable. The cis isomers have a 1.10 kcal/mol stability penalty in the Benson heat of formation group additivity dataset. 1,2-difluoroethylene, 1,2-difluorodiazene (FN=NF), and several other halogens- and oxygen-substituted ethylene’s are exceptions to this norm. The cis isomer is more persistent than the trans isomer in these situations. The cis effect is the name for this occurrence.
Significance of geometrical isomerism NEET exam
Geometrical isomerism contributes to roughly 4% of the total number of questions answered in the last 30 years, as per the NEET Chapter Wise Weighting factors Organic Chemistry – Some Basic Principles and Methods chapter. It may be beneficial to create a schedule and set out a time for additional scoring sections. If the themes with the highest weightage are chosen, the NEET correction cycle will be accelerated up. As a result, this article briefly discusses the notion of isomerism and its various kinds.
Crack NEET with Result-Oriented Learning Program from Infinity Learn
FAQs
Stereoisomerism occurs when the atoms that make up an isomer are linked in the same sequence but have a distinct spatial arrangement. Stereoisomerism can occur in several ways, one being geometric isomerism.
Cis isomers are almost always polar, whereas trans isomers are almost never. Trans isomers have a low polarity. Non-polar molecules make up a large proportion of trans isomers. Trans isomers have lower melting points than cis isomers due to their loosely packed molecules. The melting temperatures of trans isomers are frequently greater than those of cis isomers due to the tightly-packed molecules.
The spatial arrangement of atoms differs amongst geometrical isomers. Geometrical isomers have the same formula, but their atoms or groups are arranged differently in space. Chemical and physical qualities differ between them. What are stereoisomers, and how do they work?
What is the variation amongst trans and cis isomers?
Do geometrical isomers differ in (a) functional group position (b) atom position (c) atom spatial arrangement (d) carbon chain length?








