Table of Contents
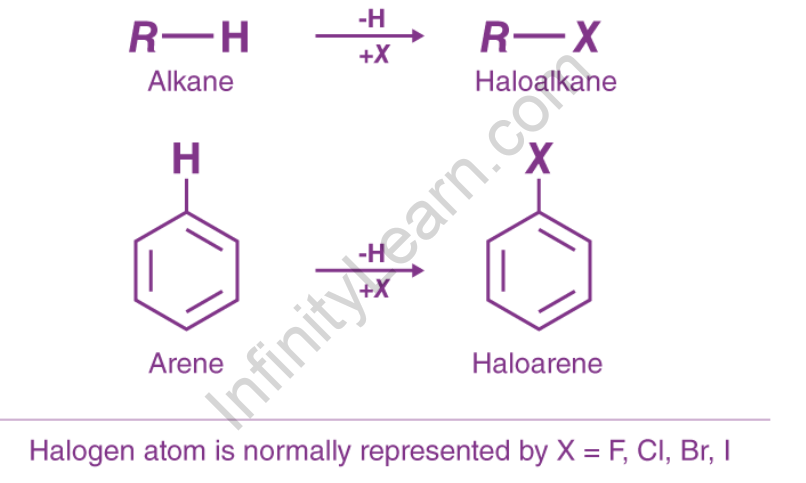
Haloalkanes and haloarenes are hydrocarbons that contain one or more halogen atoms in place of one or more hydrogen atoms. The compound formed when a hydrogen atom is replaced by an aliphatic hydrocarbon containing a halogen atom is known as haloalkane. Other names for the same thing include alkyl halide and halogenoalkane. When a hydrogen atom is replaced by a fragrant hydrocarbon, the resulting halogen atom compound is known as haloarene. Also referred to as aryl halide or halogenoarene. The halogen group is represented by X in haloalkane (R – X). It is attached to the alkyl group’s sp3 composite atom, whereas in haloarene (Ar – X), the halogen is attached to the aryl group’s sp2 composite atom. Bromobenzene is a Haloarenes example.
Haloarenes are aromatic hydrocarbon halogen derivatives in which the halogen atom is directly attached to a carbon atom of the aromatic ring. They are also known as aryl halides. When a halogen atom replaces a hydrogen atom attached to an aromatic ring, haloarenes are formed.
Ar–H+X→Ar–X+H
Haloarene has the general formula Ar–X, where Ar represents an aryl group and X is a halogen atom.
The primary distinction between haloalkanes and haloarenes is that haloalkanes are formed from open-chain hydrocarbons (alkanes), whereas haloarenes are formed from aromatic hydrocarbons (it is a type of hydrocarbon that due to sigma bonds and delocalized pi electrons between carbon atoms, it forms a circular or ring-like structure)
Overview
Haloalkanes and haloarenes are hydrocarbons in which one or more hydrogen atoms have been replaced by halogen atoms. The primary distinction between haloalkanes and haloarenes is that the former is derived from open-chain hydrocarbons (alkanes), whereas the latter are derived from aromatic hydrocarbons.
The systematic names of haloarenes or aryl halides are derived by prefixing the aromatic hydrocarbon name with fluoro, chloro, bromo, or iodo. In disubstituted or trisubstituted compounds, the relative positions of the substituent groups are denoted by Arabic numerals. The series is numbered in such a way that it yields the lowest number sequence. In the case of disubstituted derivatives, the relative positions 1,2,1,3, and 1 can be represented by the prefixes ortho (O–), meta (m–), and para (p–). Because the C–O bond in phenols has a partial double bond character due to resonance, it is stronger and more difficult to cleave. As a result, haloarenes cannot be produced from phenols.
Arenes : Haloarenes can be produced by direct halogenation of benzene in the presence of a halogen carrier or Lewis acids such as FeCl3, AlCl3, and others. In addition to these, iodine and iron filings can be used as halogen carriers. The reaction, which involves electrophilic substitution, is catalysed by resonance stabilised carbocation.
In general, halogen atoms are attached to sp3 hybridised carbon atoms in haloalkanes, whereas sp2 hybridised carbon atoms are attached to haloarenes. The difference in the hybridization state of the carbon atom in the C-X bond is responsible for the two families’ distinct characteristics. Haloalkanes and haloarenes are more chemically reactive than parent alkanes and aromatic compounds due to the presence of halogens. These compounds have a variety of medicinal applications as well.
Haloarenes example
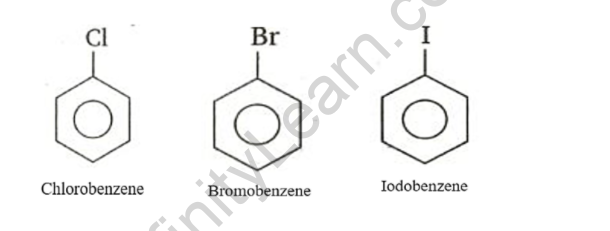
Haloarenes are aromatic alkane hydrocarbons with one or more hydrogen atoms replaced by halogens. Aromatic hydrocarbons are haloarenes. These are made by halogenating aromatic rings directly. These are hydrocarbons with a closed chain. These compounds have a pleasant aroma. In substitution reactions, haloarenes do not precipitate. Haloarenes are compounds formed when hydrogen atoms attached to benzene rings are replaced by halogen atoms. Examples include chlorobenzene, bromobenzene, iodobenzene, 2-chlorotoluene, and other haloarenes.
Haloarenes meaning
Aryl Halides/Haloarenes/Halogenoarene are aromatic compounds in which a halogen group replaces one or more hydrogen atoms attached to an aromatic ring. Haloarenes differ from haloalkanes primarily in their preparation method and properties. This compound class and its derivatives are extremely diverse and serve a variety of functions. Aryl chlorides are an important member of the haloarene class. Haloarenes are also hydrocarbons made up of aromatic rings that have one or more hydrogen atoms replaced by halogens. The halogen atom is attached to the sp3 hybridised carbon atom of the alkyl group in haloarenes.
Haloalkanes and haloarenes are used in a variety of industrial and everyday applications. They are used in a variety of applications, including flame retardants, propellants, solvents, pharmaceuticals, refrigerants, fire extinguishants, and many others. They are used as non-polar compound solvents. These compounds’ derivatives are used in medicine, for example, chloramphenicol is used to treat typhoid fever. Malaria patients are treated with synthetic halogen compounds such as chloroquine. DDT is used as an insecticide.
Haloalkanes and haloarenes exercise solutions
Q. Why isn’t sulphuric acid used during the alcohol-Kl reaction?
Solution:
H2SO4 is a powerful oxidising agent. As a result, when used in the presence of KI, it tends to convert KI to HI and then oxidises it to I2.
Q. Arrange each set of compounds in ascending order of boiling point.
(i) Bromoform, chloromethane, and dibromomethane.
(ii) 1 chloropropane, 1 isopropyl chloride, 1 chlorobutane
Solution:
The boiling points of organic compounds are determined by the strength of their intermolecular forces. These forces are van der Waals forces (a) and dipole-dipole interactions (b). These forces are determined by the molecules (i) molecular mass and (ii) surface area.
(i) As the compound’s molecular mass increases, so does its boiling point. As a result, the correct sequence is chloromethane, bromomethane, dibromomethane, and bromoform.
(ii) The boiling point of molecules with the same mass is determined by the size of the molecule. Branched compounds are more compact and thus have less surface area than straight chain counterparts, resulting in a lower boiling point. The boiling points are as follows: isopropyl chloride 1-chloropropane 1-chlorobutane
3. In the dark, a hydrocarbon C5H10 does not react with chlorine, but in bright sunlight, it produces a single monochloro compound C5H9Cl. Determine the hydrocarbon.
Solution:
The hydrocarbon with the molecular formula C5H10 can be cycloalkane or alkene. Because the compound does not react with Cl2 in the dark, it cannot be an alkene and must instead be a cycloalkane. Because cycloalkanes react with Cl2 in the presence of bright sunlight to form a single monochloro compound, C5H9Cl, all ten hydrogen atoms in the cycloalkanes must be equivalent. As a result, the cycloalkane is cyclopentane.
FAQs
What are the reactions of haloalkanes?
In the presence of completely anhydrous ether, haloalkanes react with magnesium metal to form organomagnesium halide, also known as Grignard reagents. In the presence of aqueous alkali solution or moist silver oxide solution, haloalkane undergoes nucleophilic substitution reaction with alcohols.
Which of the following are examples of Haloarenes?
Haloarenes are compounds formed when hydrogen atoms attached to benzene rings are replaced by halogen atoms. Examples include chlorobenzene, bromobenzene, iodobenzene, 2-chlorotoluene, and other haloarenes.
What causes Haloarenes to be more stable than Haloalkanes?
Haloarenes are more stable because they can donate their lone pair of electrons for resonance within the rings.






