Table of Contents
Important Topic of Chemistry: Markovnikov’s Addition
The configuration of the electrophilic addition of hydrogen halides to asymmetric alkenes or alkynes wherein hydrogen itself adheres to the least-substituted carbon atom in a double bond is assigned by the Markownikov or Markovnikov’s rule (or triple bond). Furthermore, hydrogen attaches to the carbon atom with additional substituents during the hydroboration of an alkene or alkyne. The rule asserts that when a protic acid HX or another polar reagent is added to an asymmetric alkene, the acid hydrogen (H) or electropositive portion attaches to the carbon with more hydrogen substituents, while the halide (X) group attaches to the carbon with more alkyl substituents.
A Brief Outline
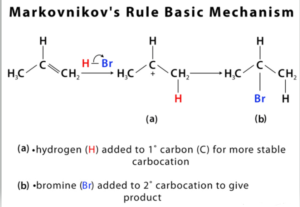
The development of the most stable carbocation during the addition process is the chemical basis for Markovnikov’s Rule. Adding a hydrogen ion to an alkene creates a carbocation with a positive charge. Due to inductive and hyperconjugation, the more replaced the carbocation is, more the stable it is. The more stable intermediate will produce the majority of the addition reaction’s products. Hydrogen is less substituted, X more substituted in main product of adding HX to alkene. At some concentrations, a less stable carbocation develops and produces a minor product with the opposite attachment of X.
Important concepts
Anti Markovnikov’s rule definition:
- Pathways that do not include a carbocation intermediate, such as free radical addition, may react by various mechanisms with different regioselectivities than those specified by Markovnikov’s rule.
- Anti-Markovnikov reactions are so named because the halogen adds to the less altered carbon, which is the polar opposite of a Markovnikov reaction. When hydrogen bromide is added to isobutylene in the vicinity of benzoyl peroxide or hydrogen peroxide, the anti-Markovnikov rule is demonstrated.
- In the research of free-radical additions, the reaction of HBr with substituted alkenes was archetypal. The unexpected presence of free radical ionizing chemicals such as peroxides was recognized by early chemists to be the cause of the fluctuation in the ratio of Markovnikov versus anti-Markovnikov reaction products.
- The O-O bond in peroxides is relatively weak, which explains why. The O-O bond can be broken into 2 radicals with the help of light, heat, or even just operating on its own.
- After that, the radical groups can interact with HBr to form a Br radical, which acts with the double bond. Because the bromine atom is so big, it is more likely to come into contact with and react with the least substituted carbon, resulting in fewer static interactions between carbon and the bromine radical.
- Furthermore, the radical species is most stable when the unpaired electron is in the more substituted location, analogous to a positively charged species. Hyperconjugation stabilizes the radical intermediate. More carbon-hydrogen bonds are oriented with the radical’s electron-deficient molecular orbital in the more substituted position.
- This indicates that there are more hyperconjugation effects, making that position more advantageous.
Markovnikov’s and Anti markovnikov’s rule
-
Rule of Markovnikov
In addition to processes involving alkenes or alkynes, Markovnikov’s rule states that the proton is added to the carbon atom with the most hydrogen atoms attached.
-
The Anti-Markovnikov Rule
In addition to alkenes or alkynes, the proton is transferred to the carbon atom with the least number of hydrogen atoms linked to it, according to the Anti Markovnikov rule. The Anti Markovnikov rule, also known as the peroxide effect or Kharasch effect, acts against the Markovnikov rule.
Difference between Markovnikov’s and Anti Markovnikov’s rule
To make chemical compounds, we use chemical processes. We can get the desired product if we have the right number of reactants and catalysts and other circumstances, such as the right temperature. However, the chemical reaction may not always produce the desired compound, or it may produce a combination of chemicals that includes the desired product as well as others. The Markovnikov rule can be used to describe this circumstance. The Markovnikov law expresses why a specific element or group is connected to a specific carbon atom in a molecule rather than any other carbon atom. The Anti Markovnikov rule illustrates the inverse of the Markovnikovs rule’s circumstance.
Markovnikov’s rule attaches H to more substituted C, whereas Anti-Markovnikov attaches H to less substituted C.
Significance of Markovnikovs addition in NEET exam
Chemistry requires a strong understanding to succeed in tests. Preparing for a subject without enough study materials and solutions, on the other hand, can be challenging. Referring to chemistry answers and practising will help students ace the NEET. Infinity Learn’s solutions help pupils self-analyze and progress in CBSE syllabus areas.
FAQs
Question 1: What does Markovnikov’s rule say about the future?
Answer: Protic acid HX addition to alkene follows Markovnikov’s rule. The protic acid’s halide will attach to highly substituted carbon atoms, with hydrogen attaching to the least replaced carbon.
Question-2: Which of the reactions defies Markovnikov’s rule?
Answer: Free radical addition reactions do not follow Markovnikov’s rule, as regioselectivity is not predicted by it. Anti-Markovnikov addition reactions are the most common name for these reactions.
Question-3: Is bromination a reaction of addition or subtraction?
Answer- Any process or reaction that involves bromine being introduced into a molecule. Bromination occurs when Br2 is electrophilically added to an alkene. A benzene ring is brominated by electrophilic aromatic substitution. To bromate a benzylic location, a free radical substitution method is used.









