Table of Contents
Introduction
An orbital is a mathematical statement that explains the behavior of no more than two electrons in the proximity of an atomic nucleus or a system of nuclei such as a molecule in chemistry and physics. An orbital is frequently depicted as a three-dimensional region in which the electron has a 95% chance of being found. Atomic orbitals are commonly denoted by a combination of numerals and letters representing specific properties of the electrons associated with the orbitals, such as 1s, 2p, 3d, and 4f. The digits, known as principal quantum numbers, represent energy levels as well as the relative distance from the nucleus.
A 1s electron lives in the energy level closest to the nucleus. A 2s electron, which is less strongly bound, spends the majority of its time away from the nucleus. The orbital shape is denoted by the letters s, p, d, and f. (The shape is determined by the magnitude of the electron’s angular momentum as a result of its angular motion.) The nucleus is at the center of as orbital, which is spherical.
A 1s electron is thus almost entirely confined to a spherical region close to the nucleus, whereas a 2s electron is restricted to a slightly larger sphere A p orbital has the shape of a dumbbell or a pair of lobes on opposite sides of the nucleus. In a p orbital, an electron has an equal chance of being in either half. The other orbitals have more complicated shapes. Before the relationship between spectra and atomic electron configuration was known, the letters s, p, d, and f were used to classify spectra descriptively into series called sharp, principal, diffuse, and fundamental.
There are no p orbitals in the first energy level, but there are three in each of the higher levels. These triplets are oriented in space as if they were on three axes at right angles to one another and can be distinguished by subscripts such as 2px, 2py, and 2pz. There are five d orbitals in all but the first two principal levels and seven f orbitals in all but the first three principal levels, all with complicated orientations. Because of their spin, each orbital can only have two electrons. An electron can be thought of as having either a clockwise or counterclockwise spin about its axis, transforming it into a tiny magnet. Electrons in full orbitals are paired with spins or magnetic polarities that are opposite.
Orbits and orbitals are not the same things: Orbit is a well-defined circular path in an atom in which electrons move around the nucleus, whereas orbitals are the three-dimensional space around an atom’s nucleus with the highest probability of finding an electron. The nodal plane is the place where the chances of finding an electron are nil. Only the s orbitals are non-directional among all the orbitals. The orbital 1s contain the most energy. The following is the distinction between a shell and an orbital: A shell in an atom is a collection of sub-shells of the same quantum number theory, n, whereas orbitals are made up of two electrons each.
Overview
In Quantum Mechanics and Chemistry, an orbital is a mathematical function that illustrates the wave-like behaviour of an electron pair, electron, or nucleon. Orbitals are also known as electron orbitals or atomic orbitals. Atomic orbitals are three-dimensional regions of space surrounding an atom’s nucleus. Atomic orbitals enable atoms to form covalent bonds. S, p, d, and f are the most often filled orbitals. According to the Pauli Exclusion Principle, no orbital space can contain more than two electrons.
Atomic orbitals are classified into several types, including s, p, d, f, g, and h. However, only the first four orbitals mentioned will be occupied on an atom’s ground state. The orbitals and their shapes are explained in detail below: The number of orbitals of a type within a subshell is given by the total values permitted form for a given value of I. The four types of atomic orbitals correspond to l values of 0, 1, 2, and 3. The orbitals with the value l = 0 are known as s orbitals, and they have a spherically symmetrical shape. It is most likely that the electron will be discovered in the nucleus. The orbitals with the value l= 1 are the p orbitals, which have a nodal plane that includes the nucleus and thus form a dumbbell shape. The d orbitals have complex shapes with at least two nodal surfaces and have l= 2 orbitals. The orbitals with l= 3 are referred to as the more complex f orbitals. Because an electron’s energy is determined by its average distance from the nucleus, each atomic orbital with a given set of quantum numbers will have specific energy associated with it, which is known as the orbital energy.
E=Z2/n2 Rhc
The distribution of orbitals into their inner electronic core is referred to as orbital penetration. Example: The radial density of the 2s orbital is spread across the curve of the 1s orbital. Similarly, 3s orbital will be divided into 1s and 2s orbitals. It will not be completely screened by the inner 1s electrons from the nucleus due to electron spreading in 2s or 3s orbitals derived from s. The extent of penetration decreases from s orbitals to f orbitals.
Concept of shapes of s,p, and d orbitals
The amount of energy of any given electron is determined by its location within an atom. Electrons exist in different energy levels, or shells, that surround the atom’s nucleus at different distances. Each shell is further subdivided into increasing energy sublevels denoted by the letters s, p, d, and f.
These sublevels are made up of orbitals, which are specific regions of space within the sublevel where an electron is most likely to be found. Orbitals are classified into four types based on their energy sublevels: s, p, d, and f. Based on the energy of its electrons, each orbital type has a distinct shape. The shape of the s orbital is spherical. The p orbital is shaped like a dumbbell. Three p orbitals differ in their orientation along a three-dimensional axis. There are five d orbitals, four of which are unique and have a clover shape with different orientations.
An orbital is a region of space around the nucleus where the chances of finding an electron of a given energy are greatest. The shape of the orbital is determined by the shape of this region (electron cloud). The shapes of orbitals are determined by the plot of angular wave functions or the square of angular wave functions (probability functions). The differences between these two plots are minor.
s orbital shape
When l = 0, the value of m is 0, suggesting that s-orbitals can only have one orientation. This means that the probability of finding an electron at a given distance from the nucleus is the same in all directions. As a result, its shape should be spherical. As a result, all s- orbitals orbiting the nucleus are spherically symmetrical and non-directional.
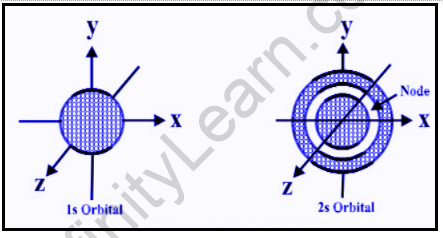
The size of an s-orbital is determined by the value of the primary quantum number n. The larger the value of ‘n,’ the larger the orbital. The 2s-orbital has an important feature in that it has a spherical shell within it where the probability of finding the electron is zero (nearly). This is referred to as a node or nodal surface. There is one spherical node in the 2s orbital. The number of nodal surfaces or nodes in any energy level’s s-orbital is equal to (n-1), where n is the principal quantum number.
p orbital shape
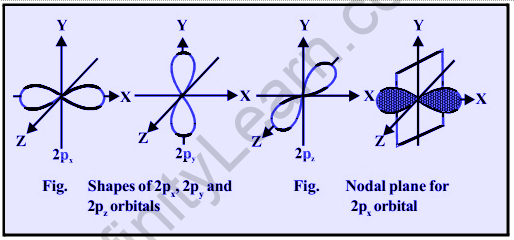
There are three values of m for p-subshell l = 1, namely -1, 0, and +1. It follows that p orbitals can have three different orientations. The energies of these three p-orbitals are the same (degenerate state), but their orientations differ. Each p-orbital is made up of two symmetrical lobes around a central axis. The lobes are denoted as 2px, 2py, and 2pz depending on their orientation, as they are symmetrical about the X, Y, and Z – axes, respectively. The cross-section of the three-dimensional boundary surface of p-orbitals is represented by the lines in the figure. The boundary surface is defined as the surface that encloses 90% of the dots representing electrons. A nodal plane separates the two lobes of each p-orbital (a plane having zero electron density). For instance, in a 2px orbital, the YZ plane is the nodal plane x. As a result, p-orbitals have a dumbbell shape and a directional character. The chances of finding the electron are equal in both lobes. Higher energy level p-orbitals have similar shapes but are larger in size.
d orbital shape
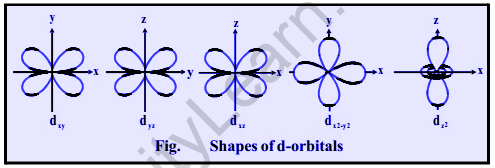
For d-subshell, l = 2, there are five possible values of m: -2, -1, 0, 1, 2. This means that d- orbitals can have five different orientations.
These are denoted by dxy,dyz dzx,dx2–y2, and dz2 respectively, planes
The dz2 orbital has a dumb-bell shape with a doughnut-shaped electron cloud in the center and is symmetrical about the Z-axis. The dx2-y2 orbital is also clover leaf-shaped, but its leaves run along the X and Y axes.
The presence of four lobes in any orbital is due to the fact that d – orbitals have two nodes, resulting in two changes in the algebraic sign of, resulting in four lobes.
FAQs
What causes the s orbital to be spherical?
All s orbitals have spherical symmetry and are spherically formed. That is, the function of the wave will be decided purely by its distance from the nucleus, rather than its direction. The size of an orbital reduces as the central quantum number of a particle decreases, but the shape stays spherical.
What does p orbital mean?
The letters s, p, d, and f stand for sharp, primary, diffuse, and fundamental, respectively. The letters and words correspond to the visual impression created by the fine structure of the spectral lines as a result of the initial relativistic corrections, particularly the spin-orbital interaction.









