Table of Contents
Introduction
Oxoacids: An oxyacid, also known as an oxoacid or ternary acid, is acid-containing oxygen. It is a compound containing hydrogen, oxygen, as well as at least just one element, with at least one hydrogen atom bound to oxygen and capable of dissociating to create the acid’s H+ cation and anion. All acids, according to Lavoisier’s original hypothesis, contained oxygen, which was termed after the Greek (oxys: acid, sharp) and the root -v (genes – creator). After it was discovered that some acids, including hydrochloric acid, lacked oxygen, acids were separated into oxo-acids and all these new hydro acids.
A brief outline
As with binary nonmetal hydrides, the acidic hydrogen is bonded to an oxygen atom in all oxyacids, hence bond strength (length) is not a consideration. Oxyacid acidity is determined by the electronegativity of the core element (X) and the number of O atoms. Acid strength grows as the number of oxygens connected to the core element X increases. Acid strength grows as the electronegativity of X increases with the same number of oxygens around E. Oxyacids are generally less stable than salts of their deprotonated forms, the oxyanions, and many of them only exist technically as hypothetical species or only exist in solution and can’t be isolated in a pure state.
There are a number of reasons for this: (1) They may condense to form oligomers or dehydrate completely to create anhydrite, (2) they may be imbalanced to one chemical of greater oxidation state and another of lower oxidation state, or (3) they may exist almost totally as another, more stable tautomeric form, phosphonic acid. Perchloric acid (HClO4), sulphuric acid (H2SO4), and nitric acid (HNO3) are a few typical oxyacids that can be manufactured as pure chemicals very simply. In an oxyacid, =O is replaced with =NR to make imidic acids.
Important concepts
Oxoacids of sulphur
Oxoacids are acids that have oxygen in them. Sulphur is recognized to create a variety of oxoacids, including H2SO4, H2SO3, and others. When sulphur is coordinated to oxygen in oxoacids, it takes on a tetrahedral form. Sulphur oxoacids usually have at least one S=O bond and an S-OH bond. In addition to S=S and S-OH, there exist terminal peroxide groups, terminal S=S, terminal and bridging oxygen atoms, and chains of (-S-) n.
- Sulphuric acid (H2SO4)
Sulphuric acid is among the most often used sulphur oxoacids. It’s a type of diprotic acid (it ionizes to give two protons). One atom of sulphur is connected to two hydroxyl groups in sulphuric acid, while the remaining two oxygen atoms form pie connections with the sulphur atom. As a result, sulphuric acid has a tetrahedral shape.
- Sulphuric acid (H2SO3)
Sulphuric acid is a diprotic acid, meaning it ionizes two protons at the same time. One sulphur atom is connected to two hydroxyl groups in sulphurous acid, and one oxygen atom forms a pie connection with the sulphur atom. Sulphur dioxide is dissolved in water and makes it.
- Peroxodisulphuric acid (H2S2O8)
Sulphur in the +6-oxidation state is present in Peroxodisulphuric acid. As a result, it is a powerful oxidizer and highly combustible in nature. Marshall’s acid is the common name for it. It has one peroxide group, which acts as a link between the two Sulphur atoms. Other than the peroxide group, each Sulphur atom is coupled to one hydroxyl group (S-OH bond) plus two oxygen atoms (S=O bond).
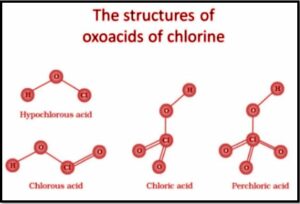
Oxoacids with chlorine
A compound with at least one oxygen, hydrogen, and at least one other element is known as an oxoacid. At a minimum, one hydrogen molecule is linked to oxygen in these. This hydrogen can split into the respective acid’s H+ cation and anion. Hypohalous acid (HOX), halos acid (HOXO), halic acid (HOXO2), and phthalic acid are some of the numerous forms of oxoacids formed by halogens (HOXO3). Only in aqueous solutions or even in the form of their salts are these oxoacids stable. The acidic strength of oxoacids increases as the number of halogens oxidized increases. Due to the absence of unoccupied d-orbitals, fluorine is an exception to this characteristic, as it only produces one oxoacid, HOF.
In a nutshell, an oxoacid is a substance that comprises at least one oxygen, hydrogen, and another element. In an aqueous solution, one hydrogen atom is bonded to the oxygen, and this hydrogen can split into the acid’s hydrogen cation and anion. Hypohalous acid, halos acid, malic acid, and phthalic acid are examples of oxoacids formed by halogens. Fluorine, unlike the other halogens, only forms one oxoacid, HOF, due to the lack of unoccupied d-orbitals.
Oxoacids with nitrogen
The alchemists of the eighth century called nitric acid, HNO3, “aqua fortis” (strong water). It is created when nitrogen dioxide (NO2) and dinitrogen pentoxide (N2O5) combine with water. After thunderstorms, small amounts of nitric acid are discovered in the atmosphere, and its salts, known as nitrates, are extensively found in nature. Massive concentrations of sodium nitrate, often known as Chile saltpeter, can be discovered in the desert along the Chilean-Peruvian border.
HNO, or nitrous acid, is a type of nitrogenous acid.
It is a weak, weak acid that can only be obtained in an aqueous solution. It’s made by acidifying a nitrite aqueous solution or dissolving an equimolar combination of NO and NO in water. Iodides to iodine, ferrous salts to ferric, anxious to stannic, and sulphites to sulphates are all converted by nitrous acid and nitrites.
HNO, or nitric acid, is a kind of nitric acid.
Nitric acid is one of the most important acids in modern chemistry. Since the thirteenth century, it has been known as a caustic solvent for metals. The Ostwald method is now almost solely used to produce HN03.
Significance of oxoacids in NEET exam
Organic, inorganic, and physical chemistry are the three areas of the Chemistry question paper. The three papers are given equal weight. Knowing what types of questions are asked on tests might help you optimize your study habits and develop new study strategies. Solving prior year’s question papers has been proven to help students engage with and comprehend a wide range of topics. Organic chemistry accounts for roughly 34% of the entire weightage.
Also read: Important Topic of Chemistry: Halides
FAQs
The electronegativity of the core atom determines the oxyacid's strength. Perchloric acid is the strongest oxyacid, whereas Hypochlorous acid is the weakest. The total number of oxygen atoms bonded in the structure is the only difference between these acids. As a result, if one acid has a higher number of oxygen atoms than another acid with a lower number of oxygen atoms, it is stronger.
Oxyacid is a type of oxygen-containing chemical molecule. In other terms, they are oxygen-carrying acids, also referred to as ternary acids or oxoacids. To be more exact, oxyacid is made up of oxygen, hydrogen, and another element, with the oxygen atom bonding with the hydrogen atom to form the acid anion and the H+ (hydrogen) cation.
In oxoacids, the center atom is sp3 hybridized. There is just one X-OH bond in every oxoacid. Most oxoacids, on the other hand, have X=O linkages. In nature, the double bond between oxygen and halogen is d pi-pi. The first member of the oxoacid family has high acidic strength. This is owing to the halogen atom's high electronegativity and small size. With a rise in the oxidation number of halogens, the acidic strength increases. What is the most powerful and least powerful oxyacid?
What is an oxyacid, exactly?
What are the structures of Halogen Oxoacids?








