Table of Contents
Phenol is a kind of aromatic chemical. This organic molecule has the chemical formula C6H6O. Carbolic acid is another name given for phenols.
It is made up of a hydroxyl group and a phenyl group that is linked together. It dissolves quickly in water. It was once used as carbolic soap. It has a slight acidic taste and is corrosive to the respiratory tract, eyes, and skin.
Phenol is a crystalline substance that is white in colour and should be handled with caution since it can cause chemical burns. Phenol was discovered by Friedlieb Ferdinand Runge in 1934 . It was made from coal tar. It is sometimes referred to as phenolic acid. If a substance has a six-membered aromatic ring and is directly attached to a hydroxyl group, it is referred to as phenol.
Types of phenols
Phenols are classified into the following groups based on the number of hydroxyl groups (OH) present:
- Monohydric phenols: They only have one OH group. The most basic component of the family is hydroxybenzene, sometimes known as phenol, while others are referred to as substituted phenols.
- Dihydric phenols: These phenols have two OH groups.
- Trihydric alcohols: Three OH groups are present in this kind of phenol.
Structure of phenol
The –OH group is connected to the sp2 hybridized carbon of an aromatic ring in phenols. The length of the C–O bond in phenol (136 pm) is somewhat shorter than that of methanol. This is owing to I the partial double bond character of oxygen generated by the conjugation of an unshared electron pair of oxygen with the aromatic ring and the sp2 hybridized state of carbon to which oxygen is linked. Because of the repulsion between two lone pairs of electrons on oxygen, the COH bond angle in alcohols is somewhat smaller than the tetrahedral angle (109°-28′).
Nature of Phenols
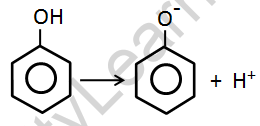
Phenoxide is formed when phenols react with active metals such as sodium and potassium. This interaction of phenol with metals suggests that it is acidic.
Phenols, like aqueous sodium hydroxide, react to form phenoxide ions. This suggests that phenols have a greater acidity than alcohol and water molecules.
The Acidity of Phenols: An Explanation
- The capability of phenols to lose hydrogen ions and produce phenoxide ions accounts for their acidity.
- The sp2 hybridized carbon atom of the benzene ring connected directly to the hydroxyl group works as an electron-withdrawing group in a phenol molecule.
- The electronegativity of this sp2 hybridized carbon atom of a benzene ring connected directly to the hydroxyl group is greater than that of the hydroxyl group.
- The electron density on the oxygen atom lowers due to the increased electronegativity of this carbon atom in contrast to the hydroxyl group connected.
- The reduction in electron density raises the polarity of the O-H bond, resulting in increased phenol ionization.
- As a result, the phenoxide ion is generated. The formation of the phenoxide ion is stabilized by the delocalization of the negative charge caused by the resonance in the benzene ring.
Preparation of phenol
The following are general methods for phenol preparation:
- Derived from sulphonic acids (by alkali fusion of sodium benzene sulphonate): The first commercial phenol production technique. At 573K, sodium benzene sulphonate is fused with sodium hydroxide to form sodium phenoxide, which is acidified to yield phenol.
C6H5SO3Na+2NaOH→ C6H5ONa +Na2SO3+H2O
2C6H5ONa+2HCl → 2C6H5OH+2NaCl
- Phenol Synthesis from Diazonium Salts: Diazonium salts are formed when an aromatic primary amine is fused with nitrous in the presence of HCl(NaNO2 + HCl) acid at 273 – 278 K. In nature, these diazonium salts are exceedingly reactive. When heated with water, these diazonium salts hydrolyze to phenols. By treating diazonium salts with dilute acids, phenols can be obtained.
- Phenol Synthesis from Haloarenes: Chlorobenzene is an example of a haloarene formed via mono substitution of the benzene ring. At 623K and 320 atm, chlorobenzene reacts with sodium hydroxide to generate sodium phenoxide. Finally, when sodium phenoxide is acidified, it produces phenols.
- Cumene-based Phenol Preparation: Cumene is a natural product formed via the Friedel-Crafts alkylation of benzene with propylene. Cumene hydroperoxide is formed through the oxidation of cumene (isopropylbenzene) in the presence of air. When cumene hydroperoxide reacts with dilute acid, phenols are formed.
- Dow Chemical Process: Chlorobenzene is reacted with dilute sodium hydroxide at around 300°C and 3000 psi pressure in this method.
The Use of Phenols
Some of the uses of phenol are given below.
- The transformation of phenol to plastics precursors is one of the most important applications of phenol, accounting for two-thirds of its production.
- Bisphenol-A, a major precursor to epoxide resins and polycarbonates, is produced during acetone condensation.
- Phenol is also a helpful precursor to a wide range of medications, most notably aspirin, but also a number of herbicides and pharmaceutical pharmaceuticals.
- Phenol is a component of the liquid/liquid phenol-chloroform abstraction process, which is used in molecular biology to obtain nucleic acids from tissues or cell culture materials.
- Condensation of alkylphenols, phenols, or diphenols with formaldehyde produces phenolic resins, one of which being Bakelite.
- Because phenol is so inexpensive, it has many small-scale applications. It is used in industrial paint strippers in the aviation sector to remove polyurethane, epoxy, and other chemically resistant coatings.
- Phenol byproducts have been utilized in the production of cosmetics such as hair dyes, sunscreens, skin lightening preparations, and skin toners or exfoliators.
- When phenol is partially hydrogenated, it produces cyclohexanone, a precursor to nylon. Nonionic detergents are made by alkylating phenol to produce alkylphenols, such as nonylphenol, which are subsequently ethoxylated.
FAQs
What is the use of phenol?
Because phenol is so inexpensive, it is used in a wide range of small-scale applications. This compound is found in industrial paint strippers used in the aviation industry to remove epoxy, polyurethane, and other chemically resistant coatings. Phenol derivatives can be found in cosmetics such as sunscreens, hair colorants, skin lightening preparations, and skin toners/exfoliators.
What is carbolic acid?
Carbolic acid is another name for phenol. It has the chemical formula C6H5OH and is an aromatic organic molecule.
Is phenol basic or acidic?
Phenol is classified as a weak acid. In aqueous solutions of pH 5-6, it is in equilibrium with the phenolate anion C6H5O (also known as phenoxide). The presence of an OH group in phenol makes it more acidic than aliphatic molecules, while the aromatic ring resonance stabilizes the phenoxide anion.









