Table of Contents
In valence bond theory, resonance, also known as mesomerism, is a way of defining bonding in specific molecules or ions by combining many contributing structures. It has a special meaning when it comes to expressing delocalized electrons inside certain compounds or polyatomic ions when the bonding can’t be stated by a single Lewis structure. Resonance is an expansion of the concept that the bonding in a chemical species can be characterized by a Lewis structure, according to valence bond theory. A single Lewis structure, composed of atoms satisfying the octet rule, potentially having formal charges, and joined by bonds of positive integer order for multiple chemical species.
A brief outline
Isomerism and resonance are not the same things. Isomers are chemical entities having varied configurations of atomic nuclei in space that share the same chemical formula. A molecule’s resonance contributors, on the other hand, can only change how electrons are explicitly assigned to atoms in Lewis’s structure representations of the molecule. When a molecular structure is described as being given by a resonance hybrid, this does not imply that the molecule’s electrons are “resonating” or changing back and forth between numerous sets of locations, each of which is defined by a Lewis structure.
Rather, it denotes that the collection of contributing structures is an intermediate structure with a unified, well-defined geometry and electron distribution. Even if the term “resonance” may conjure up such an image, it is erroneous to think of resonance hybrids as fast interconverting isomers. In a discussion of the quantum states of the helium atom in 1926, Werner Heisenberg brought the resonance mechanism into quantum mechanics. He compared the helium atom’s structure to a conventional system of resonating linked harmonic oscillators.
Important concepts
Structural resonance
- Because of the complex architecture of the casing and supporting elements, structural resonance is the more prevalent resonant situation. The structure anchoring a machine or a non-spinning machine component is usually resonant at or near the machine’s rotating speed.
- Even minor vibratory pressures caused by the machine’s residual imbalance and misalignment effects can excite the resonant base structure, causing significant vibration.
- The reed frequency vibration that frequently occurs with vertical turbine pumps with a motor situated on top of the discharge elbow is a prime illustration of structural resonance. Resonant machine components are also possible.
- The force is constant in structural resonance, while the vibratory output of the changes occurs with speed. Natural frequencies and mode shapes will exist in any structural system with mass and rigidity.
- The response of the structure will be enhanced if it is dynamically aroused at or near any of these frequencies, resulting in higher than predicted vibration and stress levels, perhaps up to 40 or 50 times higher than if the same excitation force was delivered statically.
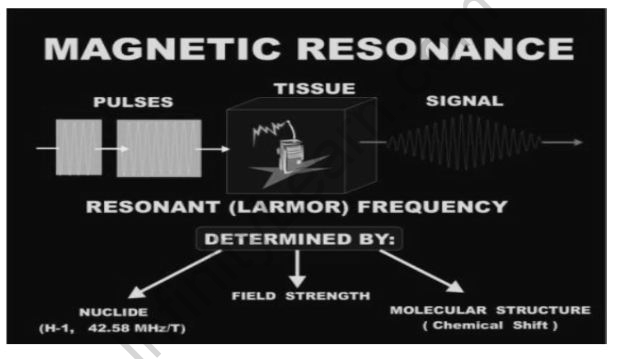
Nuclear magnetic resonance
Nuclei in a strong constant magnetic field are agitated by a weak oscillating magnetic field and respond by creating an electromagnetic signal with a frequency representative of the magnetic field at the nucleus. When the oscillation frequency equals the intrinsic frequency of the nuclei, which is determined by the intensity of the static magnetic field, the chemical nature, and the magnetic characteristics of the isotope, this process occurs near resonance.
Due to its chemical shift of the resonant frequency of the nuclear spins in the sample, NMR spectroscopy is one of the most used techniques for obtaining physical, chemical, electrical, and factors – about molecules. Peak splitting between nuclei caused by J- or dipolar couplings is also beneficial. The functional groups, topology, kinetics, and three-dimensional structure of molecules in solution and solid-state can all be studied using NMR spectroscopy. Peak integrals can be used to assess composition quantitatively because the area under an NMR peak is usually proportional to the number of spins involved.
Nuclear quadrupole resonance
NQR (nuclear quadrupole resonance spectroscopy) is a nuclear magnetic resonance-based chemical analysis technology (NMR). NQR spectroscopy is considered as “zero Field NMR” because it can detect nuclei transitions in the absence of a magnetic field, unlike NMR. The interplay of the electric field gradient (EFG) with the quadrupole potential of the nuclear charge distribution causes the NQR resonance.
In contrast to NMR, NQR absorption occurs in the absence of an applied magnetic field. When an external static field is applied to a quadrupolar nucleus, the quadrupole levels are separated by the energy predicted by the Zeeman interaction. The nature and symmetry of the interaction around the nucleus are critical to the approach. When done at different temperatures, it can characterize phase transitions in solids. Because the shifts in the liquid phase are averaged to zero due to symmetry, NQR spectra can be studied for solids.
 Significance of resonance in NEET exam
Significance of resonance in NEET exam
The NEET topics are intended to clarify and present the most probable questions on the exam. Observations from experts in the subject, which are available for free on the Infinity Learn website, could be used to clarify them in plain English. Multiple-choice questions are simple to practice if students have a good understanding of the topics presented during the program.
Examine the thorough notes carefully to ensure that you grasp this topic, as this will help you prepare for the NEET exam. It’s also a good idea to jot down some thoughts on resonance for later study.
Also read: Important Topic of Chemistry: Covalent Bond
Frequently Asked Questions
Question: What techniques are used in NMR Spectroscopy?
Answer: The following are the numerous NMR Spectroscopy techniques:
- When the NMR active nuclei are placed in a magnetic field, they absorb energy at a resonant frequency.
- A nuclear magnetic resonance response is achieved in this way of acquiring spectra. It emits a faint signal that radio receivers can only detect with high sensitivity.
- A radio frequency emitter, a powerful magnet containing a spinning sample container, and a receiver with a probe are all used in an NMR spectrometer to perform NMR spectroscopy.









