Table of Contents
Introduction:
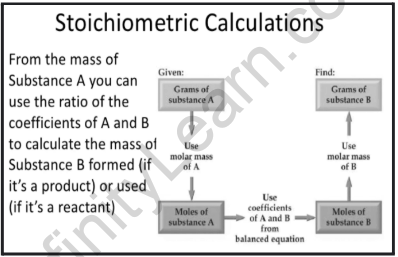
Stoichiometric calculations: A balanced equation indicates the reactants and products, and also meets the requirements of molecules or moles consumed or supplied for each. Stoichiometry is the term used to describe the quantitative correlations between the masses, moles, and particles (atoms, molecules, and ions) of the products and reactants in a balanced chemical equation. The amount of product or reactant determined by the coefficients in a balanced equation is referred to as a stoichiometric quantity. To address stoichiometric problems using various conversion factors, we must follow a few steps. They are as follows:
- Equilibrium is achieved by balancing the formula given.
- Assigning a mole value to the material.
- The number of moles is calculated.
In the combustion reaction, oxygen combines with the fuel, and the stoichiometric point is the point at which all of the oxygen has been used and all of the fuel has been burned. Some of it remains unreacted when there is additional oxygen (over stoichiometric combustion). Similarly, if the combustion is partly due to a lack of oxygen, the fuel will stay unreacted. (Unreacted fuel may also remain due to sluggish combustion or inadequate mixing of fuel and oxygen; this is unrelated to stoichiometry.) The stoichiometry of different hydrocarbon fuels varies due to differences in carbon, hydrogen, and other components.
A brief outline
In a chemical reaction mechanism, the ith component’s stoichiometric number is defined as
Vi = ∆Ni/∆ ξ
where Ni is the number of molecules of i, and ξ is the extent of reaction
The degree to which a compound interacts in a reaction is represented by the stoichiometric number. The standard is to allocate negative values to reactants and positive numbers to products, following the rule that as the extent of the reaction increases, the composition shifts from reactants to products. Any reaction, on the other hand, maybe interpreted as going in the opposite direction, and therefore would alter in the negative direction in order to reduce the system’s Free energy.
The proportions of the substances existing at any given time, which affect the kinetics and thermodynamic, i.e., if equilibrium lies towards the right or left of the initial state, determine whether a reaction will actually go in the arbitrarily specified forward direction or not.
Important concepts
Because elementary reactions always involve complete molecules, stoichiometric coefficients for all step-in reaction mechanisms are always integers. Some rational fractions may be found in a composite version of an overall response. There are frequently chemical species involved that do not engage in a reaction and hence have zero stoichiometric coefficients. Any regenerated chemical species, such as a catalyst, has a stoichiometric coefficient of 0.
The term “stoichiometry” comes from two Greek words: “stanchion,” which means element, and “metry,” which means measurement. In this idea of stoichiometry, we have had the following two sub-sections.
- Analysis based on gravity
- Analysis of volumetric data
Chemical Formulas are used in Stoichiometric Calculations.
- The total atomic weights of the numerous atoms contained in the substance’s molecule is the formula mass. For example, the mass of Na2S can be calculated as, 2(23) + 1(32) = 78.
- The Avogadro number is: The overall number of particles in one mole of a substance is known as Avogadro’s number. It is the precise number of atoms found in 12g of C-12. The value of the Avogadro number is 6.022×1023
- The sum of the entire mass of all the atoms that make up a molecule per mole is known as molar mass.
- The following reaction can be used to demonstrate the mole ratio of reactants and products:
- 2Na + 2HCl + 2NaCl + H2 = 2NaCl + H2
- We could see those 2 moles of Na mixed with 2 moles of HCl in the aforementioned process. This reaction produces 2 moles of sodium chloride and 1 mole of hydrogen gas. As a result, we receive x mole of NaCl in combination with x mole of HCl for a certain amount of sodium, say x mole. x/2 moles of hydrogen gas will be created.
Stoichiometry formulas
How to convert gram to moles
Stoichiometry is utilized in conversions as well as to balance chemical equations, such as converting from grams to moles employing molar mass as the conversion factor or from grams to milliliters using a density. To calculate the amount of sodium chloride (NaCl) in 2.00 g, for example, perform the following:
2g NaCl/ 58.44 g NaCl mol-1 = 0.0342 mol
The molar ratio
Chemical equations are frequently balanced using stoichiometry (reaction stoichiometry). In an exothermic process, the two diatomic gases hydrogen and oxygen, for example, can merge to produce a liquid, water, as stated by the equation:
2H2 + O2 = 2H2O
The molar proportions of components in stoichiometric compounds are also referred to as stoichiometry (composition stoichiometry). The stoichiometry of hydrogen and oxygen in H2O, for example, is 2:1. The molar proportions of stoichiometric compounds are full integers.
Calculating the quantity of product
Stoichiometry could be used to figure out how much of a product a reaction produces. A piece of solid copper (Cu) introduced to an aqueous solution of silver nitrate (AgNO3) would replace the silver (Ag) in a single displacement process, yielding aqueous copper (II) nitrate (Cu (NO3)2) and solid silver. When 16.00 grams of Cu are added to a solution of extra silver nitrate, how much silver is produced?
The steps would be as follows:
Write the equation and make sure it is balanced.
- Moles to mass: To convert grams of copper to moles of copper, use the formula below.
- Mole to mole ratio: Convert the number of moles of Cu produced to the number of moles of Ag produced.
- Mole to the mass ratio: Calculate the number of grams of Ag produced from a mole of Ag.
Significance of chemical reactions in NEET exam
As we’ve all heard, “continue to rehearse.” This is similarly valid for Class 12 understudies. Understudies can accomplish amazing outcomes on their board assessments assuming that they apply what they’ve realized. This generally permits understudies to find their solid and flimsy spots, yet it likewise assists them with having a less focused outlook on their tests.
In a standing wave, the objective of NEET subjects is to clarify and introduce the most probable inquiries that will show up in the test. These can be clarified in straightforward terms involving notes from experienced scholastics in the field, which are accessible on the Infinity Learn online stage. Different decision questions are easy to respond to on the off chance that understudies have a decent comprehension of the subjects educated all through the program.
Also read: Important Topic Of Physics: Dalton’s atomic theory
FAQs
What is the definition of a limiting reagent?
In a chemical calculation, limiting reagent is a crucial notion. A specific chemical reaction refers to a reactant that is contained in a minimum stoichiometric quantity. In the chemical reaction, this particular reactant is completely consumed. As a result, we use the limiting reagent to perform all computations linked to various products or in a series of reactions.
What is the application of stoichiometry?
A chemical equation that is balanced informs us of the exact number of quantities (called moles) that are involved in a chemical reaction. We can compute the number of invisible parties required for practical or analytical purposes using these numbers.
How do you address difficulties involving mass-to-mass stoichiometry?
The mass-mass stoichiometry computation entails translating the reactant's mass into moles of reactants, and then determining the mole of the product using the mole ratio, which is then converted to mass. First, we must balance the chemical equation to answer the mass stoichiometry problem. The mole ratio can be calculated with the use of a balanced chemical equation.









