Table of Contents
Introduction:
Coulomb’s law or Coulomb’s inverse square law is an empirical law of physics. It quantifies the magnitude of the force between two stationary and charged particles.
The electric force that exists between charged objects at rest is commonly referred to as the Coulomb force or electrostatic force.
The magnitude of the electrostatic force between fixed charges is always described by Coulomb’s law. This is necessary for the development of electromagnetic theory.
This might be a key point because nowadays it is possible to discuss the quantity of electric charge in a meaningful way.
The information about Coulomb’s law from various physics-related articles is available here. Coulomb’s law and its general concepts are important topics in physics. Students who want to flourish in physics need to be well known about the term Coulomb’s law to get deep knowledge about it to do well on their exams and it is very much useful in real life. The definition, forms, and limitations are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional physics help.
Overview:
In 1785, a French physicist named Charles Augustin de Coulomb was the first to formulate a tangible relationship between two charged bodies in mathematical form. He also published an equation for the force that objects attract or repel each other, known as Coulomb’s law or Coulomb’s inverse square law.
According to Coulomb’s law, the attractive or repulsive force between two charged objects is directly proportional to the product of the charges and inversely proportional to the square of the distance between them. It acts along a line connecting two charges, which are considered point charges.
Coulomb’s law gives an idea of the force between two point charges. The word “point charge” means in physics that the magnitude of linear charged bodies is very small compared to the distance between them.
So, we consider them as point charges as it is easy to calculate the attractive/repulsive forces between them.
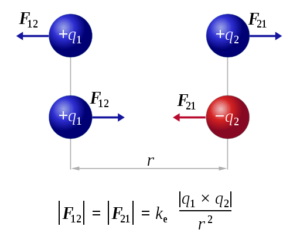
Coulomb’s Law Formula:
Scalar Form:
The Coulomb’s Law in scalar form will be,
F=K e q 1 q 2 ⁄r 2
Where ‘k e ‘ is the constant of the Coulomb’s Law k e ≈8.99×109 N⋅m²C-2,q 1,q 2 are the charges’ signed magnitudes, and the scalar ‘ r ‘ is the distance between the charges.
Then, the interaction force among the charges is attractive if the charges have opposite signs and it will be repulsive if like-signed.
Vector Form:
The Coulomb’s Law in vector form will be,
F 1 2=1/4πε0. q 1 q 2 ⁄r 1 22 . rˆ12
F 12 =-F 21
Here, F 12 is the exerted force by q 1 on q 2, whereas, F 21 is the force exerted by q 2 on q 1. Coulomb’s Law accounts for stationary charges, which are only point-sized. It is given as follows Newton’s Third Law.
F‾12=-F‾21
So, the force on a charged particle or substance because of several point charges is the net result of forces due to the individual point charges.
That is,
F‾=F 1+F 2+F 3+…
Properties of Coulomb’s Law are as follows:
In Coulomb’s Law, one of the point charges tend to deploy a force on the other point charge in order to satisfy the properties such as:
- The force which is involved will either be repulsive or attractive.
- The force will be directed towards the line that joins the two particles imaginarily.
- The like charges tend to repel whereas opposite charges tend to attract each other.
- The charges that are participated will either be negative or positive but they can never be neutral.
- The force magnitude will be directly proportional to the magnitude of the two charges which are interacting.
- The force is likely to be inversely proportional to the square of the distance among the two particles or substances that are interacting.
- When there are several point charges that are part of the interaction, then each of the point charges tends to deploy an individual force on the other point charges which is regardless of the neighbouring charges.
Coulomb’s Law – Conditions for Stability:
When ‘ q ‘ is displaced slightly towards A,FA increases in magnitude while FB decreases in magnitude.
The total force on ‘ q ‘ is directed towards A, and so it will not return to its original position. Thus, the equilibrium is unstable for axial displacement.
When ‘ q ‘ is displaced perpendicular to AB, then the force FA and F B bring the charge to its original position. Thus, for a perpendicular displacement, the equilibrium is stable.
1 Coulomb of Charge:
A coulomb is a charge, that repels the same charge of the same sign having a force of 9×10 N while the charges are apart one meter in a vacuum and the Coulomb force is the conservative internal and mutual force.
The value of 0 is given by,
8.86×10-12 C²/ N m²
OR
8.86×10-1 2 F m-1
Finally, we have seen that the Coulomb force will be true only for the static charges.
Limitations of Coulomb’s Law are as follows:
- Coulomb’s Law will be applicable only for the point charges which are at rest.
- This law can only be applied in the cases where the inverse square law is followed.
- It is very difficult to execute this law in which the charges are in arbitrary shape. So, it is impossible to calculate the distance between the charges.
- The law cannot be used directly to determine the charge on the big planets.
Frequently Asked Question (FAQs):
Question 1: What is the meaning of absolute permittivity in Coulomb’s law?
Answer: We know that electromagnetic, absolute permittivity is said to be a measure of the resistance that occurs when an electric field is formed in a medium.
In other words, it can also be expressed as measuring how an electric field affects and is affected by a dielectric medium in which it is present. The dielectric constant is also directly related to electrical susceptibility, which also measures how easily a dielectric polarizes in response to an electric field around it.
Question 1: What are the similarities between the electrical and gravitational forces with regards to Coulomb’s law?
Answer: Both forces are electric and gravity is a non-contact force. It can be seen that Coulomb’s law equations for electric force are very similar to the gravitational equations and that the two are almost identical. Both equations show the inverse quadratic relationship between the force and the distance between the two bodies involved. And these two questions also show that force is proportional to the product of the quantity that produces it. On the other hand, in the case of gravity, it is mass, and in the case of electric force, it is a charge.
Question 3: What are the applications of Coulomb’s law?
Answer: The applications of Coulomb’s law are as follows:
- To determine the force and distance between the two charges.
- To determine the electric field.









