Table of Contents
The Davisson-Germer experiment was an experiment designed to measure the thermal conductivity of a metal and is considered one of the most famous experiments in physics. The experiment was conducted by Robert A. Millikan and Harvey Fletcher in 1927 at the California Institute of Technology. It is often cited as an example of how particle physics can be used to test hypotheses about macroscopic objects with relative ease. The experiment consisted of a thin, flat metal disk that was immersed in a glass tube filled with oil, surrounded by water on all sides except for one side where the oil meets air. The disk had two electrodes attached to it that were connected to a high voltage power supply, which created an electric field across the gap between them and caused a constant current to flow through both electrodes into the surrounding water.
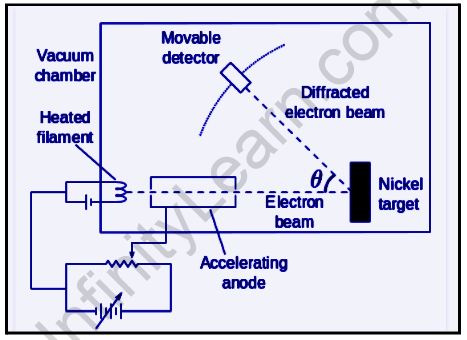
The Davisson–Germer experiment was conducted by Clinton Davisson and Lester Germer at Western Electric (later Bell Labs) between 1923 and 1927,[1] in which electrons scattered by the surface of a nickel metal crystal displayed a diffraction pattern. This experiment confirmed Louis de Broglie’s hypothesis of wave-particle duality, which he proposed in 1924, and was a watershed moment in the development of quantum mechanics.
Walter M. Elsasser in Göttingen in the 1920s made an important contribution to the Davisson–Germer experiment by remarking that the wave-like nature of matter could be investigated by electron scattering experiments on crystalline solids, just as the wave-like nature of X-rays had been confirmed by X-ray scattering experiments on crystalline solids. Elsasser’s suggestion was then relayed to physicists in England by his senior colleague (and later Nobel Prize winner) Max Born. When the Davisson and Germer experiment was carried out, the results were explained by Elsasser’s proposition. The Davisson and Germer experiment, on the other hand, was designed to study the surface of nickel rather than to confirm the de Broglie hypothesis.
Scientists’ initial atomic models could only explain the particle nature of electrons but failed to explain the properties related to their wave nature. In 1927, C.J. Davisson and L.H. Germer performed an experiment known as the Davisson Germer experiment to explain the wave nature of electrons through electron diffraction. In this article, we will learn about the experiment’s observations and conclusions.
The Davisson-Germer experiment is a famous thought experiment in physics. It is a way of understanding the nature of time. In this experiment, an electron is fired at a metal plate with a single small hole that emits light. The electron passes through the hole and creates an interference pattern on the back wall of the box. The frequency of light emitted from one side of the box would be shifted by an amount proportional to the speed of light in relation to that side. The shift would be related to how long it takes for the electron to pass through the hole and reach its destination on the other side. For the first time, the Davisson and Germer Experiment established the wave nature of electrons and validated the de Broglie equation. In 1924, De Broglie proposed the dual nature of matter, but it wasn’t until later that Davisson and Germer’s experiment confirmed the findings. The discoveries provided the first experimental proof of quantum mechanics. In this experiment, we will look into the scattering of electrons by a Ni crystal. The Davisson-Germer experiment demonstrated the electron’s wave nature, confirmed deBroglie’s earlier hypothesis. It was a significant step forward in the development of quantum mechanics because it put wave-particle duality on a firm experimental footing. The Bragg law for diffraction had previously been applied to x-ray diffraction, but this was the first time it had been applied to particle waves.
What is Davisson and Germer experiment?
A vacuum chamber is used for the Davisson and Germer experiment. As a result, the medium’s electron deflection and scattering are avoided. The following are the main components of the experimental setup:
- A low voltage power supply was used to heat an electron gun with a tungsten filament F coated with barium oxide.
- When a suitable potential difference is applied from a high voltage power supply, the electron gun emits electrons that are then accelerated to a specific velocity.
- The released electrons were directed toward the surface of a nickel crystal by passing through a cylinder perforated with small holes along its axis, resulting in a fine collimated beam. As a result, electrons scatter in various directions.
- The intensity of the electron beam produced is measured by the electron detector, and it is then moved on a circular scale after being connected to a sensitive galvanometer (to record the current).
- The intensity of the scattered electron beam is measured for different values of angle of scattering by moving the detector on the circular scale at different positions that change the (angle between the incident and scattered electron beams).
What did Davisson-Germer experiment prove?
- An electron cannon was heated with a tungsten filament F coated with barium oxide using a low voltage power supply.
- When an appropriate potential difference from a high voltage power source is applied, the electron cannon produces electrons that are then accelerated to a specific velocity.
- These liberated electrons were forced to pass through a cylinder perforated with small holes along its axis, resulting in a finely collimated beam.
- The beam from the cylinder is directed once more toward the surface of a nickel crystal. As a result, electrons disperse in a variety of ways.
- The electron detector records the intensity of the generated electron beam, which is then moved on a circular scale after being linked to a sensitive galvanometer (to record the current).
- The intensity of the scattered electron beam is measured at various angles of scattering by moving the detector on a circular scale at various locations that modify the (angle between the incident and scattered electron beams)
Davisson and Germer experiment related to wave nature
The Davisson and Germer experiment demonstrated the wave nature of electrons, confirming de Broglie’s earlier hypothesis. When electrons are scattered from crystals with appropriately spaced atoms, they exhibit diffraction. The Davisson and Germer experiment assumed that waves reflected from two distinct atomic levels of a Ni crystal would have a fixed phase difference. Following reflection, these waves will interact in either a constructive or destructive manner. As a result, a diffraction pattern appears. In the Davisson and Germer experiment, waves were used instead of electrons. These electrons drew together to form a pattern of diffraction. The dual nature of substance was thus established. As shown below, the de Broglie equation and Bragg’s law are related:
We have, according to the de Broglie equation:
=h/p
=h/V(2mE)
=h/(2meV)
As a result, an electron has a wavelength specified by the equation for a given V.
The following equation expresses Bragg’s Law:
nλ=2 d sin(90°- θ/2)
Also read: Rutherford’s Model of the Atom
FAQs
Which of the following is used in the Davisson-Germer experiment? In the Davisson – Germer experiment, which crystal is used?
Nickel crystal was used in the Davisson – Germer experiment. The surface of the nickel crystal is bombarded with a fine beam of electrons. As a result, the electrons are scattered in all directions by the crystal's atoms.
What was the outcome of Davisson and Germer's experiment?
The Davisson-Germer experiment concluded that electrons have a wave nature as well, thereby supporting De Broglie's theory of wave-particle duality of matter. In this experiment, accelerated electrons fired from an electron gun were used to bombard a nickel crystal in a vacuum.

