Table of Contents
Magnetism is concerned with the study of properties mediated by magnetic fields. Electric currents and elementary particle magnetic moments combine to form a magnetic field, which acts on other currents and magnetic moments. Magnetism is a component of the larger phenomenon of electromagnetism. When placed in an external magnetic field, all matter exhibits magnetic properties. Even materials such as copper and aluminium, which are not typically thought to have magnetic properties, are affected by the presence of a magnetic field produced by either pole of a bar magnet. The matter is classified as paramagnetic or diamagnetic depending on whether the pole of a magnet attracts or repels it. The most well-known effects occur in ferromagnetic materials, which are strongly attracted by magnetic fields and can be magnetised to become permanent magnets, thereby producing magnetic fields. Demagnetization of a magnet is also possible. Only a few materials are ferromagnetic; the most common are iron, cobalt, nickel, and their alloys. Less common examples include the rare-earth metals neodymium and samarium. Because permanent magnetism was discovered in lodestone, a type of natural iron ore known as magnetite, the prefix ferro- refers to iron.
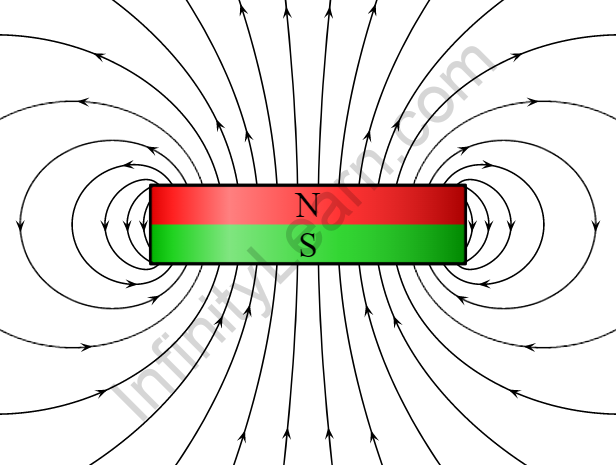
Overview
Magnetism can be found in all substances. The bulk susceptibility of magnetic materials is classified. Although ferromagnetism is responsible for the majority of the effects of magnetism encountered in everyday life, there are several types of magnetism. Paramagnetic materials, such as aluminium and oxygen, are weakly attracted to a magnetic field; diamagnetic materials, such as carbon and copper, are weakly repelled; and antiferromagnetic materials, such as spin glasses and chromium possess a more complex relationship with a magnetic field. The force of a magnet on diamagnetic, paramagnetic, and antiferromagnetic materials is usually too weak to be felt and can only be detected by laboratory instruments, so these substances are frequently referred to as non-magnetic in everyday language. Generally, the magnetic state (or magnetic phase) of a material is affected by temperature, pressure, and the magnetic field applied. As these variables change, a material may exhibit more than one type of magnetism. Although, the exact mathematical relationship between strength and distance varies, the strength of a magnetic field almost always decreases with distance. Magnetic fields can become complicated as a result of different configurations of magnetic moments and electric currents. While some theories predict the existence of magnetic monopoles, only magnetic dipoles have been observed.
Electrical and magnetic properties
The transfer of an electrical charge from one position to another is referred to as the conduction of electricity through a material. The charge can be transferred via electron movement or ion migration. Conduction in metals is caused by electron migration, but ion movement is responsible for electrolyte conductivity and the extremely low conductivities observed in some insulating materials. A charge is carried by the motion of electrons and the movement of positive ‘holes’ in the opposite direction in the class of materials known as semiconductors.
Electrical conduction in metals is caused by electron migration through the crystal lattice, and metals are classified as good conductors due to high electron mobilities. A metal’s resistivity rises as its temperature rises. The vibrational amplitude of the atoms increases as the temperature rises, increasing the likelihood of interference and collision between moving electrons and atoms. However, increasing the pressure effectively increases the number of electrons per unit volume of material, lowering the resistivity. This property is useful, and electrical strain gauges are built on it.
As in the case of ionic conductivity, an increase in temperature increases ion mobility, resulting in an increase in conductivity. The effect of heat on an electron conductor is the inverse of this.
Magnetic properties
Each substance in our environment contains magnetic properties. In the presence of a magnetic field, different materials exhibit different properties. The magnetic properties of a substance are caused by electrons in the atoms or molecules. Every electron in an atom acts like a miniature magnet. Electrons are also known as small current loops that retain their magnetic moment.
These magnetic moments result from two types of electron motion:
- An atom’s orbital movement around its nucleus.
- When an electron revolves around its own axis.
A system’s magnetic moment quantifies the strength and direction of its magnetism. The term is usually used to refer to the magnetic dipole moment. A magnetic moment can be found in anything magnetic, such as a bar magnet or an electric current loop. A magnetic moment is a two-dimensional vector quantity with a magnitude and a direction. An electron has an electron magnetic dipole moment, which is caused by the electron’s intrinsic spin property, and this causes the electron to be an electric charge in motion. There are several types of magnetic behaviour, such as paramagnetism, diamagnetism, and ferromagnetism.
The ability of transition metals to form magnets is an intriguing property. Unpaired electron metal complexes are magnetic. Because the final electrons are in the d orbitals, this magnetism must be due to unpaired d electrons. A single electron’s spin is denoted by the quantum number ms as +(1/2) or –(1/2). When an electron is paired with another, its spin is negated, but when the electron is unpaired, it creates a weak magnetic field. The paramagnetic effects are amplified as the number of unpaired electrons increases. In a coordination compound, the electron configuration of a transition metal (d-block) changes due to repulsive forces between electrons in the ligands and electrons in the compound. The compound may be paramagnetic or diamagnetic depending on the strength of the ligand.
Magnetic material
Magnetic materials were those that a magnetization state can be induced. These materials generate a magnetic field in their surroundings. The response of a material to an external magnetic field is at the heart of magnetism. The material’s spinning electrons act like tiny magnets. These tiny magnets are aligned in the direction of the applied magnetic field, magnetising the material.
The word magnet is typically reserved for objects that produce their own persistent magnetic field even in the absence of an applied magnetic field. This is only possible with certain types of materials. Most materials, on the other hand, generate a magnetic field in response to an applied magnetic field – a phenomenon known as magnetism. Magnetism can be classified into several types, and all materials exhibit at least one of them.
The total magnetic behaviour of a material can vary greatly depending on its structure, especially its electron configuration. Various types of magnetic behaviour have been observed in various materials.
Paramagnetic
The magnetic state of an atom with one or more unpaired electrons is referred to as paramagnetism. Due to the magnetic dipole moments of the electrons, a magnetic field attracts unpaired electrons.
The paramagnetic material is a material or body that is attracted very weakly by the poles of a magnet but does not retain any permanent magnetism. It has a slightly higher relative permeability than unity and is slightly magnetised. They only have a weak attraction to the lines of forces. Aluminium, chromium, manganese, lithium, magnesium, and other metals are examples.
Paramagnetism is most common in substances where some or all of the individual atoms, ions, or molecules have a permanent magnetic dipole moment. The magnetization of such matter is determined by the magnetic energy to thermal energy ratio of the individual dipoles. The Brillouin function, which depends only on the ratio (B/T), can be used to calculate this dependence in quantum theory. At low magnetic fields, magnetization is linearly proportional to the field and reaches its maximum saturation value when the magnetic energy exceeds the thermal energy by a large factor.
There is an additional contribution to susceptibility in substances with a nuclear magnetic dipole moment. The nuclear magnetic moment is only about one thousandth the size of an atom.
Each occupied electron state in a normal metal has two electrons with opposite spin orientations. This is due to quantum mechanics’ Pauli exclusion principle, which allows for no more occupancy of energetically preferred states. However, in the presence of a magnetic field, it is more energetically favourable for some of the electrons to move to higher states. The electron moments in these states can be oriented along the field despite the presence of only single electrons. The resulting paramagnetic susceptibility is temperature independent. The temperature has no effect on net susceptibility. Because the diamagnetic and paramagnetic contributions are of comparable magnitudes, a metal’s net susceptibility can be of either sign.
FAQ’s
How do you determine the magnetic properties of a material?
Materials' magnetic properties are primarily determined by the magnetic moments of their atoms' orbiting electrons. The magnetic moments of atom nuclei are typically thousands of times smaller than the magnetic moments of electrons, making them insignificant in the context of material magnetization.
Is magnetism a physical property?
Magnetism is a physical entity because attracting something to a magnet does not cause the substance to change (change in composition) and does not involve chemical reactions.
Is gold magnetic?
Gold was long thought to be a nonmagnetic metal. However, scientists recently discovered that gold can be magnetised by applying heat. Gold was long thought to be a non-magnetic metal. However, Tohoku University researchers recently discovered that gold can be magnetised by applying heat.









