Table of Contents
A semiconductor is a silicon-based material that conducts electricity better than an insulator like glass but not as well as a pure conductor like copper or aluminum. The introduction of impurities, known as doping, can change their conductivity and other properties to match the unique needs of the electrical components in which they live. Semiconductor devices can exhibit various valuable characteristics, including changeable resistance, the ability to pass current more rapidly in one direction than another, and the ability to react to light and heat. Their primary functions are signal amplification, switching, and power conversion.
A brief outline
They’re used in nearly every industry, and the companies who make and examine them are seen to be strong indicators of the general economy’s health. Semiconductors arrive in a range of sizes.
Types of semiconductors
Semiconductors are divided into four major product categories:
-
Memory
Memory chips are transitory data storage devices that transport information to and from the brains of computer machines. The memory business continues to consolidate, reducing memory prices so low that only a few behemoths such as Toshiba, Samsung, and NEC can finance to stay in the game.
-
Microprocessors
These are centralized processing entities that house the core logic that allows tasks to be completed. With the exception of Advanced Micro Devices, Intel’s dominance in the microprocessor segment has forced practically every other competitor out of the mass market and into lesser niches or different segments entirely.
-
Commodity integrated circuit
Commodity Integrated Circuit (CIC) is a type of integrated circuit in which these chips, sometimes known as “regular chips,” are made in large batches for normal processing. This area, which is ruled by very large Asian chip manufacturers, has razor-thin profit margins that can only be competed for by the largest semiconductor companies.
-
Complex SOC
Complex SOC “System on a Chip” is the fabrication of an integrated circuit chip that contains the capacity of an entire system. The market is driven by rising demand for consumer items with additional features at lower prices. With the markets for memory, microprocessors, and commodity integrated circuits all but closed, the SOC segment is perhaps the only one left with adequate room for a diverse set of enterprises.
Important concepts
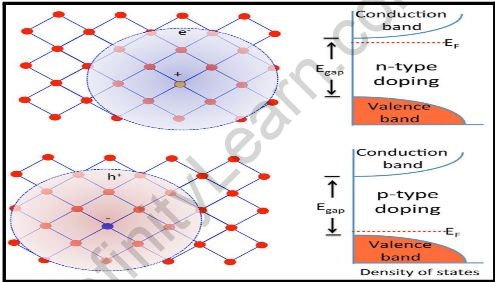
n-type Semiconductors:
- A pentavalent impurity element with five electrons in its outer shells is called a pentavalent impurity element in an N-type semiconductor. To boost the number of electrons obtainable for conduction, pentavalent defects or dopant elements are introduced to N-type semiconductors.
- Pentavalent impurity elements are doped into the N-type semiconductor. The valence shell of pentavalent elements has five electrons. Phosphorus (P), Arsenic (As), and Antimony (An) are instances of pentavalent contaminants (Sb).
- The pentavalent impurity is added to the N-type semiconductor in such a small amount that the original intrinsic semiconductor’s crystal structure is not affected. Four silicon atoms form covalent bonds with the pentavalent impurity atom, while one electron is not linked to any silicon atom.
- Donor impurities are named this because each pentavalent impurity atom provides one electron to the N-type semiconductor. As a result, the N-type semiconductor has a higher quantity of electrons.
Example of an N-type Semiconductor
An intrinsic semiconductor material such as silicon (Si) contains 14 electrons in a 2,8,4 configuration, while Germanium (Ge) has 32 electrons in a 2,8,18,4 configuration. To be stable, each atom needs 8 electrons in its valence shell. As a result, intrinsic semiconductor atoms form covalent connections by sharing electrons with a neighboring atom to attain an atomic structural balance of 8 electrons.
N-type semiconductors have a number of advantages.
The conductivity of a pure semiconductor is low. Extrinsic semiconductors, on the other hand, have a substantially higher conductivity than intrinsic semiconductors, even at room temperature. As a result, n-type semiconductors can be used at room temperature. Again, free electrons are the majority carriers in this form of semiconductor, allowing the material to behave like a typical conductor at a fixed temperature.
p-type Semiconductors:
- Suppose an electron acceptor is doped into the intrinsic semiconductor to convert it to a p-type semiconductor. By receiving an electron from the lattice, the electron acceptor is important for the process of a hole.
- As a result, the p-type semiconductor’s majority carriers are holes. In this approach, the electron acceptor capability of a p-type semiconductor is defined.
- The first stage in creating a p-type semiconductor is to dope an intrinsic semiconductor with the trivalent intruder. The valence shell of this type has three electrons and requires one extra electron.
- This is made feasible by the sharing of electrons. It is commonly referred to as an acceptor since it accepts electrons. Boron, indium, and gallium are acceptor impurities. P-type semiconductors are created when these elements are introduced to silicon or germanium.
- There are four silicon atoms adjacent to the boron, implying that there must be four electrons, but there are only three valence electrons existent. A hole is defined as the lack of the fourth electron or even the vacancy of the electron.
- It denotes that the boron atom accepts one electron. The void or requirement for an electron can be filled. The number of vacancies and the number of electrons accepted are proportional.
- Because it is important for the process of holes in semiconductors, boron is clearly classified as a trivalent impurity. As a result of doping a pure form of silicon with boron, a p-type semiconductor is formed.
Example of a P-Type Semiconductor
Trivalent impurities like boron or gallium are widely employed as doping impurities in silicon. Then boron or gallium doped silicon is a wonderful example of a p-type semiconductor. Using the same notion of boron and silicon, whether silicon is doped with gallium or indium, the process can be illustrated.
Semiconductors have a number of important properties, including:
- At zero Kelvin, a semiconductor serves as an insulator. It behaves as a conductor when the temperature goes up.
- Semiconductors can be doped to make semiconductor devices suited for energy conversion, sensors, and amplifiers due to their excellent electrical properties.
- There are fewer power losses.
- Semiconductors are smaller and lighter than transistors.
- They have a higher capacitance than conductors but less resistivity than an insulator.
- When the temperature rises, the resistance of semiconductor materials lowers, and vice versa.
Significance of conductors in NEET exam
The audit materials from Infinity Learn grant students to get to resources online at their unwinding and from the convenience of their own homes. It is made out of point-prepared experts and is therefore 100 percent exact and dependable for tests like NEET. The survey material from Infinity Learn is open for download in a pdf plan, which students can store for future reference or view in an electronic manner. The audit materials help backups in quickly settling their inquiries.
Also read: Insulators
FAQs on Semiconductors
Why would the resistivity of semiconductors drop as the temperature is raised?
The difference in charge carrier density amongst conductors and semiconductors causes the difference in resistivity. Because the quantity of charge carriers grows rapidly as temperature rises, the resistance of semiconductors lowers, making the fractional change, or temperature coefficient, negative.
At ambient temperature, why is the valence band partially vacant and the conduction band partially full in semiconductors?
At zero Kelvin, the conduction band in semiconductors is empty, and the valence band is totally filled. At this temperature, no electron from the valence band can pass into the conduction band. However, due to minor prohibited transitions, some electrons in the valence band leap across to the conduction band at ambient temperature.
How well are Semiconductors Used in Industry?
Semiconductors' physical and chemical qualities enable them to create modern marvels such as microchips, transistors, LEDs, and solar cells.








