Table of Contents
Le Chatelier’s principle, also known as Chatelier’s principle (or the Equilibrium Law), is a chemistry principle that predicts the effect of a change in conditions on chemical equilibria. The principle is named after Henry Louis Le Chatelier, a French chemist, but it is sometimes credited to Karl Ferdinand Braun, who discovered it independently. It can be stated as follows: When a system that has been in equilibrium for a long time is subjected to a change in concentration, temperature, volume, or pressure, the system changes to a new equilibrium, and this change partially offsets the applied change. The principle is commonly regarded as a more general observation of systems.
When a stable system is disturbed, it will adjust to reduce the impact of the change. Alternatively, “roughly stated Any change in the status quo triggers a counter-response in the responding system. The concept of systemic maintenance of an equilibrium state despite perturbations is known by different names depending on the discipline (for example, homeostasis, an idea that encompasses the concept, is commonly used in biology) and has been studied in a variety of contexts, most notably in the natural sciences. The principle is used in chemistry to manipulate the outcomes of reversible reactions, often to increase their yield. In pharmacology, ligand binding to receptors can shift the equilibrium according to Le Chatelier’s principle, explaining the various phenomena of receptor activation and desensitization. The principle has been generalized in economics to help explain the price equilibrium of efficient economic systems. In systems of simultaneous equilibrium, phenomena that appear to contradict Le Chatelier’s principle can also occur.
The principle of Le Chatelier is as follows: A change in one of the variables that describe an equilibrium system causes a shift in the position of the equilibrium that cancels out the effect of the change. Le Chatelier’s principle describes what happens to a system when it is temporarily thrown out of equilibrium. This section focuses on three methods for modifying the conditions of a chemical reaction at equilibrium:
(1) altering the concentration of one of the reaction’s components
(2) adjusting the system’s pressure
(3) altering the temperature at which the reaction is carried out.
Overview
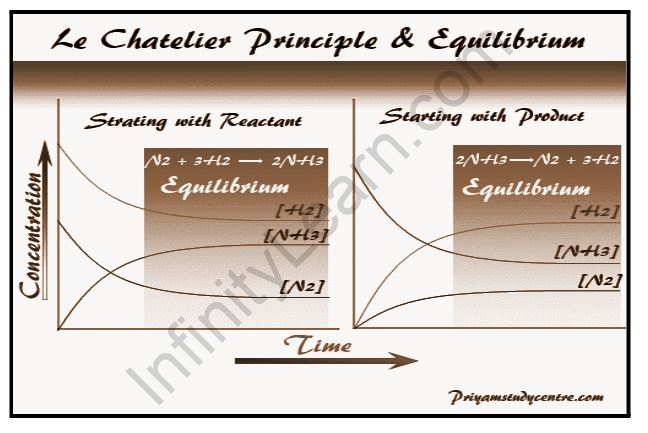
A useful guide is available to assist you in estimating how chemical equilibrium will shift in response to changes in reaction conditions, such as temperature or pressure. In 1884, the French chemist Henri Le Chatelier discovered that if an equilibrium chemical system is disturbed, the system will adjust itself to minimize the effect of the disturbance. Le Chatelier’s principle refers to this qualitative reasoning tool. The state of a chemical reaction when the forward and reverse reactions are occurring at equal rates is referred to as dynamic equilibrium, also known as a chemical equilibrium. Both the product and reactant concentrations remain constant. This does not imply that the concentrations of reactants and products must be equal; rather, neither concentration changes because the rates of formation are equal. In most cases, chemical equilibrium and dynamic equilibrium are the same, though technically there could be a case of dynamic equilibrium with no chemical changes. According to Le Chatelier’s principle, if a system in dynamic equilibrium is disturbed by a change in its conditions, the position of equilibrium will shift to compensate for the change.
Le Chatelier’s principles, also known as the equilibrium law, are used to predict the effect of changes on a chemically balanced system (such as the change in temperature or pressure). The principle is named after Henry Louis Le Chatelier, a French chemist. According to Le Chatelier, equilibrium adjusts the forward and backward reactions in order to accept changes affecting the equilibrium conditions.
State Le Chatelier’s principle
The Le Chatelier principle predicts the effect on a chemically balanced system when some of the factors such as temperature, pressure, and concentration change. As a result, Le Chatelier in 1885 and Braun in 1886 predicted how the system behaves in chemistry or chemical science if any of the system’s parameters are changed. Le Chatelier made the generalization that if a system is in equilibrium, a change in any of the factors that determine the state of equilibrium will cause the equilibrium to shift in such a way that the effect of the change is minimized.
As a result, for learning chemistry, we use the Le Chatelier principle, also known as the principle of mobile equilibrium, to express the thermodynamic effect of pressure, temperature, and concentration on equilibrium.
Le Chatelier’s principle rules
Although the equilibrium constant for the ideal gas molecule has no dimension and is independent of reaction pressure. However, Le Chatelier’s principle draws some special conclusions for reacting and product components. For example, if the volume of the non-reactive system is reduced by a certain amount, the pressure rises in proportion. As a result, the equilibrium shifted to the low volume sides, according to the Le Chatelier principle. As a result, the pressure increases are less than those of the non-reactive system. Because of this shift in the equilibrium position of natural gases, the reactive system is more compressible than the non-reactive system.
Similarly, if a fixed amount of heat is supplied to the non-reacting system, the temperature of the corresponding system increases. The heat supplied does not significantly raise the temperature. Since the equilibrium has shifted to the side of higher enthalpy or free energy. The heat generated by this shift in equilibrium is much higher than in the non-reactive system since the Le Chatelier principle chose the reacting system as a heat storage medium.
Also read: Important Topic of Chemistry: Strong Electrolyte
FAQs
What are the three factors influencing the Le Chatelier principle?
The principle of Le Chatelier can be used to predict the behaviour of a system in response to changes in pressure, temperature, or concentration.
What is the principle of Le Chatelier?
According to Le Chatelier's principle, if a reaction at equilibrium is subjected to a change in parameters such as temperature, pressure, or the concentration of reactants and products, the reaction equilibrium shifts in the direction of the change.







