Table of Contents
We can consider chemistry as the study of matter’s pattern and transformation. The substance is another term that is frequently used interchangeably with the matter, but it has a more limited definition in chemistry. Chemistry is also treated as the study of the behavior of matter. Chemistry is concerned with the composition, structure, and properties of matter, as well as the phenomena that occur when different types of matter change. Matter theory encompasses the evolving ideas and systems used to describe and explain the material world. A large part of matter theory was based on an element theory. The matter is classified into different categories based on the physical properties it exhibits and the states in which it exists; these are referred to as states of matter. Changes in the properties of matter caused by external influences such as pressure and temperature distinguish states of matter. A break in one of these characteristics frequently distinguishes states.
Overview
Knowing the distinctions between Solid, Liquid, and Gas is critical because they represent the three states of matter. As we all know, everything around us is matter, and it is critical to understand how matter is classified, primarily into Solids, Liquids, and Gases. A solid is a rigid substance with low intermolecular spacing and high intermolecular forces that binds all molecules within it together. Liquids, on the other hand, are less rigid and easy to flow. They typically have properties that allow them to flow from higher to lower levels. When compared to solids, this is a unique property of liquids.
The vapour pressure of a liquid is the spot in a closed container at which equilibrium pressure is reached between molecules leaving the liquid and entering the gaseous phase and molecules leaving the gaseous phase and entering the liquid phase. Intermolecular forces in liquids are weak. Increasing the temperature of the liquid molecules can help to break these forces and convert them to the vapour phase, increasing the liquid’s vapour pressure.
Liquid State
Fluids include both liquids and gases. The possible explanation for this is their ability to flow. A liquid, on the other hand, is not the same as a gas at the molecular level. Liquids exhibit a variety of properties and behaviours, ranging from forces of attraction to the effects of physical properties.
The following characteristics are shared by all liquids:
- Strong intermolecular forces: The intermolecular forces in a liquid are stronger than those in gas but weaker than those in a solid. The strong interaction between molecules is due to the fact that they share less space at the molecular level.
- Definite volume and density: Liquids have a defined volume. Because of the small space between the molecules, these dissimilar gases occupy a limited amount of space. A liquid’s molecules rarely separate from one another under normal physical conditions. Liquids are not only denser than gases, but they are also less compressible.
- Liquids are free-flowing and shapeless: Taking on the shape of the container in which liquids are stored. Because of the free-flowing molecules that move past each other, liquids also have a flowing characteristic.
Liquids exhibit the above-mentioned characteristics under normal temperature, pressure, and volume conditions. When physical conditions change, the basic properties of liquids change dramatically. Aside from the characteristics listed above, liquids have the following features:
- Vapour Pressure
- Boiling Point
- Surface Tension
- Viscosity
Vapour Pressure
Vapour pressure is a liquid property related to evaporation. In other words, it is the pressure exerted by vapour at a given temperature and in a closed system when it is in equilibrium with its phases, either solid or liquid. Vapour pressure is typically calculated in standard pressure units. The pascal (Pa) is the SI unit, and one pascal equals one newton per square meter.
If a container is filled with a liquid, the vapours from that liquid occupy its walls. Liquids have the unique property of transforming into vapours as the temperature rises! In general, vapours from an aqueous substance occupy the walls of the unfilled portion of the container and exert pressure on those walls; this pressure is referred to as the vapour pressure.
In the beginning, the vapour pressure rises, but after a while, it stabilises. Due to evaporation, some of the molecules in the container will be converted to vapour over time. As a result, the volume of liquid decreases while the volume of vapour increases. The evaporated gas molecules will move in a random pattern along with the empty space in the container. As a result of this random movement, some of these molecules will come into contact with the container’s uppermost layer and begin to condense. Gradually, an equilibrium between the liquid and vapour phases is reached. The equilibrium vapour pressure of saturated vapour pressure is the vapour pressure at the point of equilibrium. The entire phenomenon of vapour formation is temperature-dependent and thus tends to increase with increasing temperature.
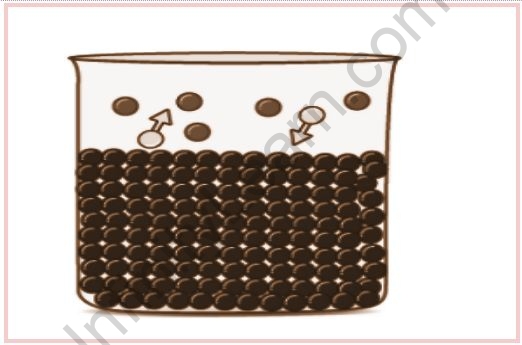
As time passes, the number of molecules in a gaseous state increases, as does the rate of condensation. It eventually reaches a point where the rate of evaporation equals the rate of condensation. This is referred to as the equilibrium stage. The pressure exerted by the molecules on the wall at this point is known as the liquid’s vapour pressure.
The factors influencing vapour pressure are:
- Nature of liquid
- Effect of temperature
- Concentration of solute
Relation Between Vapour Pressure and Boiling Point:
The relationship between vapour pressure and boiling point is inverse. If a liquid’s vapour pressure is low, its boiling point is high, and vice versa. The intermolecular forces of attraction are inversely proportional to the vapour pressure.
- Lower intermolecular forces indicate a higher vapour pressure for the liquid. The amount of heat energy required to split the molecules is reduced, and the boiling point is quite low.
- If the intermolecular forces are strong, the molecules will be strongly attracted to one another. Fewer molecules separate and condensate into vapours. Finally, the vapour pressure will be low. The boiling point will be high in this case.
Vapour Pressure of Water
The pressure exerted by molecules of water vapour in gaseous form is referred to as the vapour pressure of water (whether pure or in a mixture with other gases such as air). The pressure at which water vapour is in thermodynamic equilibrium with its condensed state is known as the saturation vapour pressure. Water condenses at pressures greater than the vapour pressure while it evaporates or sublimates at lower pressures. The Clausius–Clapeyron relation can be used to calculate the saturation vapour pressure of water as the temperature rises. The temperature at which the saturated vapour pressure equals the ambient pressure is known as the boiling point of water.
In meteorology, calculations of the (saturation) vapour pressure of water are commonly used. The temperature-vapour pressure relationship describes the relationship between the boiling point of water and pressure in reverse. This applies to both pressure cooking and high-altitude cooking. Understanding vapour pressure is also important for understanding high-altitude breathing and cavitation.
Also read: Liquefaction of Gasses
Frequently Asked Questions
What is the Difference Between Vapour Pressure and Atmospheric Pressure?
The pressure created by the earth's atmosphere at any given point is equal to the sum of the mass of the atmospheric column above that point and the gravitational acceleration at that level. This is known as atmospheric pressure. The boiling point of a liquid is directly proportional to atmospheric pressure. When a liquid attempts to escape into a gaseous state, atmospheric pressure limits its ability to do so by applying pressure to it. The pressure exerted by the vapours above the liquid is defined as the vapour pressure. The vapour pressure of a liquid is inversely proportional to its boiling point. Whenever a liquid attempts to escape into a gaseous state, the vapour pressure forces the liquid molecules apart by exerting an outward force from the liquid.
What is the property of a material which processes maximum vapour pressure?
At room temperature, the material with the lowest boiling point has the highest vapour pressure. A material with a high boiling point has a low vapour pressure.
What is the effect of boiling point in vapour pressure?
The boiling point is inverse to the vapour pressure. When a liquid is heated, more molecules enter the atmosphere, increasing the vapour pressure. In general, the boiling point of a liquid is the point at which the vapour pressure equals the atmospheric pressure.









