Table of Contents
Introduction
When studying the theory behind electrical circuits, it’s common to assume that all of the circuit’s components are perfect. The system’s battery is believed to have zero resistance in an ideal state. However, this is not the case. When working with batteries in the real world, it is discovered that they have some internal resistance, which influences the current in the circuit. It is sometimes not indicated on the battery, and as the battery empties, it changes. In that circumstance, determining the internal resistance of the battery in use becomes critical. We can say that internal resistance happens when there is current in a device or electrical circuit and a voltage drop in the source voltage or source battery. Things like electrolytic material in batteries or other power sources are the main cause of this. In general, the internal resistance can be described as the barrier to current flow supplied by the cells and batteries themselves, resulting in heat creation.
The information about internal resistance from various physics-related articles is available here. Measurement of the internal resistance of a cell is an important topic in physics. Students who want to flourish in physics need to be well known about internal resistance and its measurement to get deep knowledge about it to do well on their exams. The definition, brief explanation, and its importance are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional physics help.
Overview
Electric cells are sometimes known as “electric power supplies.” Cells produce both electricity and chemical processes. We know that the batteries are built up of one or more electrochemical cells. Electromotive Force (EMF) is a unit of charge measurement that is measured in coulombs. It is the pressure or electric intensity created by electrical energy or a source. It’s a gadget that converts any type of energy into electrical energy, which is then measured in coulombs.
A big emf-based battery is known to be larger than batteries with lower emf. Because these batteries have greater energy, they can deliver higher currents. It’s worth noting that a truck’s 12V battery can deliver more current than a motorcycle’s 12 V battery. The reason for this is that a truck’s battery has lower internal resistance than a motorcycle’s battery.
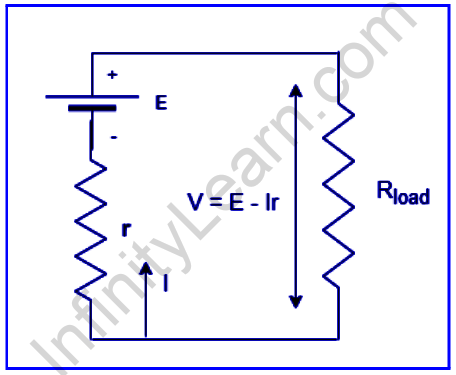
The diagram above depicts the two basic components of a voltage source. The resistance and the internal emf of the battery. The internal resistance is denoted by r, while the emf is denoted by E. They are both in a series. The battery’s internal resistance determines how much current it can give to the circuit. The internal resistance of a battery can behave in a variety of ways, with the internal resistance of the battery increasing when the battery is depleted. However, the amplitude and direction of the electric current flowing from a voltage source, as well as the temperature and composition of the battery, may all have a role.
Importance of Internal Resistance
- It is critical to understand how much Internal Resistance an electric motor or any other electrical equipment has and how it might be minimized in order to increase its efficiency.
- When studying the internal resistance of batteries, the term “internal resistance” is used. Internal resistance is a key concept in electrical engineering that can be used in a variety of projects and experiments involving electricity.
- When constructing engines for cars, trucks, or other large vehicles, internal resistance is also critical. Internal Resistance can be used to increase the performance and fuel efficiency of Internal Combustion Engines (ICE).
Measurement of internal resistance of a cell by potentiometer
The term “potential” is used to describe the difference in potential between two places. It can also be used to compare the emf. of two cells or to determine a battery’s internal resistance. On a structural level, it is a device made out of a long wire with a uniform cross-sectional area and a length of 10 metres. When utilizing a potentiometer, it is important to ensure that the wire used in the device has a uniform cross-sectional area, low resistance, and a high-temperature coefficient. The wires are stretched parallel to one another and connected in series by copper strips. The wooden board also has a metre scale connected to it.
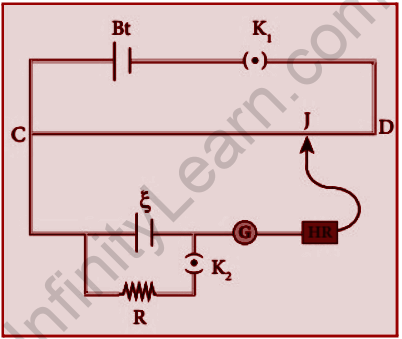
The circuit connections for measuring a cell’s internal resistance are as indicated in Figure. The positive terminal of the battery Bt is linked to the end C of the potentiometer wire, and the negative terminal of the battery is connected to the end D by a key K1. The primary circuit is formed by this.
Here, the positive terminal of the cell whose internal resistance is to be calculated is also connected to the end C of the wire. Then, the negative terminal of the cell is connected to a jockey through a galvanometer and a high resistance. Later, a resistance box R and key K2 are connected across the cell. Now, K2 is made as open, the balancing point J is obtained and the balancing length CJ=l1 is measured. Thus, the cell is in an open circuit, its emf is
ξ∝ l 1…(1)
Now, the resistance (let it be 10Ω ) is included in the resistance box and key K 2 is closed. Consider r as the internal resistance of the cell. Then, the current flowing through the cell is,
I=ξ/[R+r]
Now, the potential difference across R will be,
V=ξR[R+r]
Whenever this potential difference is balanced on the potentiometer wire, let l 2 be the balancing length.
Thus,
ξR/R+r∝ l 2…(2)
From equations (1) and (2),
R+r/R=l 1/l 2…(3)
1+r/R=(l 1/l 2)1+r/R=l 1/l 2;
r=R(l 1/l 2-1)
∴r=R( l 1-l 2/l 2)…(4)
The internal resistance of the cell can be determined by substituting the values of the R,l1, and l2. This experiment can be repeated over and over for different values of R. Finally, it can be observed that the internal resistance of the cell is not constant but increases with the increase of external resistance connected across its terminals.
Also read: Important Topic Of Physics: Calorimetry
Frequently Asked Questions (FAQs)
Question 1: What is the internal resistance of a cell?
Ans: Generally, the internal resistance can be described as the barrier to current flow supplied by the cells and batteries themselves, resulting in heat creation.
Question 2: What causes internal resistance?
Ans: We may say that the main contributors to the rise in Internal Resistance, sulfation, and grid corrosion, will lead to acid. The temperature, which influences heat resistance, lowers it while cold raises it. We may argue that heating the battery lowers the Internal Resistance for a short period of time, allowing for more runtime.
Question 3: Why is Internal Resistance important?
Ans: Internal Resistance is critical for improving the efficiency of an electric motor or any other electrical device. Knowing how much Internal Resistance a device has and how to lower it is critical. When studying the internal resistance of batteries, the term “internal resistance” is used. Internal resistance is a key concept in electrical engineering that can be used in a variety of projects and experiments involving electricity. When constructing engines for cars, trucks, or other large vehicles, internal resistance is also critical. Internal Resistance can be used to increase the performance and fuel efficiency of Internal Combustion Engines (ICE).







