Table of Contents
In chemistry, a dehydration reaction is a chemical process in which the reacting molecule or ion loses water. Dehydration reactions, on the other hand, are widespread processes that are the inverse of hydration reactions. When alcohol reacts with protic acids, it loses one molecule of water and forms alkenes. Dehydrogenation or dehydration of alcohol is the term used to describe these events. It’s an example of a reaction that eliminates something. Its rate varies depending on whether the alcohol is primary, secondary, or tertiary. The rate variation can be related to the stability of the generated carbocation. Because tertiary alcohols have more stable carbocation, they have the fastest growth of dehydration when contrasted to secondary and primary alcohols.
Alcohol and ethers have stronger Lewis bases than halide ions, owing to their leaving groups (is a halogen atom that has a negative charge). Alcohols and ethers are hence less reactive than alkyl halides. Prior to a removal or substitution reaction, they must be protonated. Because of the difficulty in producing primary carbocations, the dehydration of either a tertiary or secondary alcohol is known as an E1 reaction (two-step process), but the dehydration of primary alcohol is known as an E2 reaction (a one-step mechanism).
A Brief Outline
Dehydrogenation is the process of removing hydrogen from a feedstock, such as paraffin for olefin synthesis. The degree of dehydrogenation during thermal cracking of petroleum varies depending on the starting material and operating conditions, but because of its practical importance, methods have been developed to boost it and, in certain situations, make it nearly the only reaction. Dehydrogenation is a significant process in petroleum chemistry because it converts inert alkanes into olefins and aromatic compounds, which serve as starting points for other functional groups.
Dehydrogenation is an extremely endothermic reaction with a limited reaction to equilibrium. While establishing equilibrium or near-equilibrium while reducing side reactions and coke production are crucial features of dehydrogenation.
Important Concepts
Mechanism of acid-catalyzed dehydration of ethanol to ethene
Steps in the Dehydration Mechanism
The secondary alcohol dehydrogenation process to ketones does not occur if Raney-Ni, Al(i-ORr)3, or alumina are not present in the catalytic mixture. There is no reaction when Raney-Ni is replaced with other Ni (II) salts (e.g., NiCl2) or complexes (e.g., Ni (PPh3)2Cl2).
Alcohol dehydration is accomplished in three steps.
- Formation of protonated alcohol
- Formation of carbocation
- Formation of alkenes
- Formation of protonated alcohol: A protic acid reacts with the alcohol in this phase. It works as a Lewis base due to the obvious lone pairs just on the oxygen atom. Alcoholic oxygen undergoes protonation, making it a better leaving group. It’s a reversible process that happens swiftly.
- Carbocation formation: The C-O bond breaks in this stage, resulting in a carbocation. This is the slowest phase in the dehydration of an alcohol mechanism. As a result, the rate-determining step is the production of the carbocation.
- Alkene creation: The final step in the dehydration of alcohols is the formation of alkenes. With the help of a base, the proton created is removed. C=C is formed when the carbon atom close to the carbocation disrupts the current C-H bond. As a result, an alkene is produced.
Mechanism of dehydration of alcohol
- A dehydration reaction is a chemical reaction in which water is created by extracting water’s constituents from a single reactant. Dehydration of alcohol results in the formation of an alkene. Dehydration caused by alcohol is an illustration of an elimination reaction, which would be the polar opposite of replacement and addition reactions.
- An elimination reaction occurs when two groups or atoms on adjacent carbon atoms are eliminated or removed from a molecule, leaving numerous bonds between the carbon atoms.
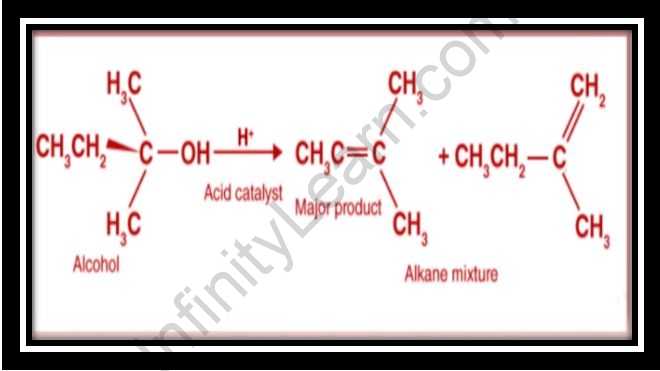
- The E1 method is used to dehydrate secondary and tertiary alcohols in acidic environments. The leaving group is successfully converted from hydroxide ion to water by protonation of the hydroxyl group. The hydronium H3O+ is much stronger than H2O, and the former H2O’s conjugate surface is a better leaving group than the latter OH’s.
- An E1 elimination might occur when a very persistent carbocation is established by dehydrating a protonated alcohol.
- Because an unstable primary carbocation is formed during E1 dehydration on primary alcohol, acid-catalyzed E1 elimination through this carbocation is extremely sluggish.
- When a proton is lost from carbon at the same time as water is lost, an E2 reaction occurs

Significance of mechanism of dehydration in NEET exam
To genius the NEET test, you ought to make sense of your topics’ significant thoughts overall. For this, answers ought to be altogether reworked and presented in a reasonable manner, using to some degree direct procedures and fewer assessments. This licenses you to save time and effort during the test. For online learning and perception of thoughts, live classes are open. Fundamental free pdfs are also open in a disengaged mode. Hence, learning and taking notes happen at the same time. By getting to these free PDFs, you can get an all-out course of action of notes.
FAQs
Alcohols dehydrate in the following order: tertiary alcohol > secondary alcohol > primary alcohol. By heating secondary and tertiary alcohols with concentrated sulfuric acid at 180°C, dilute sulphuric alcohol quickly dehydrates them. An alkene or a mix of alkenes is the result of the dehydration process of alcohol. As a result, the order of dehydration is tertiary, secondary, and primary. The order of reactivity is based on the SN1 mechanism, with tertiary alcohol reacting first with Lucas reagent.
A dehydration reaction in chemistry includes the removal of water (H2O) from the reacting molecule or ion. Condensation reactions of this type are fairly prevalent. It can be described as the hydration reaction in reverse. As a result, dehydration refers to the removal of water molecules from alcohol. All dehydration-based synthesis reactions are endothermic reactions in which smaller molecules form bonds with one another to make larger ones.
Dehydration synthesis reactions are a type of combination or synthesis reaction that involves the removal of water molecules between the same or different monomer units. It's a type of condensation reaction in which one water molecule is replaced by two others. What is the order of alcohol dehydration?
What is the dehydration process?
In a Dehydration Synthesis Reaction, what happens?









