Table of Contents
An electrophilic addition reaction in organic chemistry is an addition reaction in which a double or triple bond in a chemical compound is broken, resulting in the formation of two new bonds. The formation of an electrophile X+ that forms a covalent bond with an electron-rich, unsaturated C=C bond is the driving force behind this reaction. During the formation of the C-X bond, the positive charge on X is transferred to the carbon-carbon bond, resulting in the formation of a carbocation. The positively charged intermediate combines with an electron-rich species in the second step of electrophilic addition to form the second covalent bond. The second step involves the same nucleophilic attack as in an SN1 reaction. The precise nature of the electrophile and the nature of the positively charged intermediate are not always clear and are affected by reactants and reaction conditions. Regioselectivity is important in all asymmetric addition reactions to carbon and is frequently determined by Markovnikov’s rule. Anti-Markovnikov additions are produced by organoborane compounds. When an aromatic system is attacked by an electrophile, the result is an electrophilic aromatic substitution rather than an addition reaction.
Electrophiles are molecules that accept electrons from other compounds. In contrast, if a component donates electrons, this entity is referred to as the nucleophile. A chemical reaction between a nucleophile and an electrophile that adds to triple or double bonds is known as the electrophilic addition mechanism. An electrophilic addition reaction, in more detail, is the proclivity to combine and react with chemical substances that have a donatable electron pair (‘electron lover’). This is a fascinating and significant phenomenon in the field of organic chemistry. As a result, we will learn about electrophilic addition to alkenes and oxidising agents.
Overview
Alkenes are a class of hydrocarbons that contain at least one double bond. The alkenes undergo an addition reaction as a result of this double bond. When an electrophile attacks the double bond of carbon atoms with the help of pi electrons present in alkenes, the reaction is known as an electrophilic addition reaction of alkenes. The mechanism of the electrophilic addition reaction is explained below.
It also employs a free radical mechanism at times. Alkenes are unsaturated hydrocarbons, which means that each molecule of alkene contains at least one double bond. Because pi electrons are present, they exhibit addition reactions in which an electrophile attacks the carbon-carbon double bond to form the addition products. These are known as alkene electrophilic addition reactions. These addition reactions can also be explained by the free radical mechanism. Alkenes undergo a variety of reactions, including oxidation and ozonolysis. Several of these reactions are discussed further below:
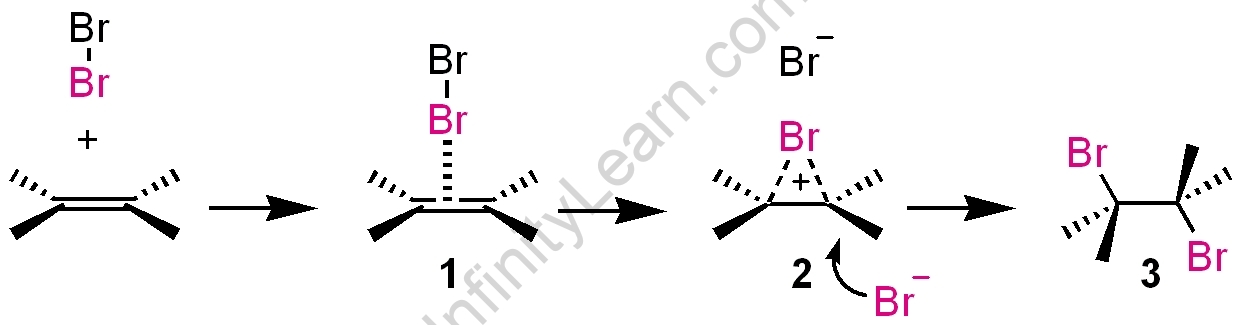
Alkene electrophilic addition reactions: Alkenes exhibit a diverse range of electrophilic addition reactions. Electrophilic addition reactions of alkenes include the addition of hydrogen halides such as hydrogen bromide and hydrogen chloride. The general trend of hydrogen halide is as follows: HI >HBr > HCl. In comparison to unsymmetrical alkenes like propene, symmetrical alkenes like ethene have a much easier time predicting the end product. As an example:
Mechanism of electrophilic addition
As previously stated, alkenes exhibit significant addition reactions. The addition of hydrogen halides in hydrogen bromide and hydrogen chloride was the simplest example for understanding the electrophilic addition reaction mechanism. Because hydrogen halides contain both protons and halides, we refer to the protons as electrophiles and the halides as nucleophiles. The first step in the electrophilic addition is to attack an electrophile on the carbon-carbon double bond, which exerts a set of electrons. This is referred to as the deprotonation step. As a result, the released electrons became attached to the molecule. It now only has a single positive-charged carbon-carbon bond. This is referred to as the carbocation process. The halide will then be attached to the carbocation, resulting in original hydrogen bonds. It will produce a new molecule if we use the correct nucleophile. In general, hydrogen halides can be represented as
HI >HBr> HCl
In such cases, Markovnikov proposed a rule known as the Markovnikov rule for predicting the major product. According to the Markovnikov rule, the negative part of the adding molecule is attached to the carbon atom with the fewest hydrogen atoms. As a result of this rule, 2-bromopropane is the expected product. This can be explained further using the mechanism of electrophilic substitution reactions of alkenes. The following is an explanation of the general mechanism: H+, an electrophile, is formed when hydrogen bromide attacks a double bond to form carbonation.
Nitration mechanism
Nitration refers to the chemical process of introducing the nitro group into an organic chemical compound. The term nitration is also used incorrectly to refer to a variety of processes, such as the formation of nitrate esters between nitric acid and alcohols, which are then used to synthesize nitroglycerin. The main difference between nitrates and nitro compounds is that the nitrogen atom forms a chemical bond with a non-oxygen atom, such as carbon or other nitrogen atoms, in the final structure. In the case of organic nitrates, the nitrogen atom is usually bonded to an oxygen atom, which is then bonded to a carbon atom.
As such, nitration is a process in Organic Chemistry that involves the replacement of a hydrogen atom (organic compound) with one or more nitro groups (single bond NO2). The reaction is usually exothermic, which means it occurs at high temperatures. Furthermore, nitration reactions can be hazardous when carried out on large scales in batches. A significant amount of heat is released, and multiple nitrations can occur, which can be extremely dangerous. As a result, the nitration reaction is usually controlled by some kind of systematic cooling that is specifically designed to remove the excess energy generated. Furthermore, the ability to control exotherms inflow improves selectivity and safety. As a result, many nitration reactions have been carried out using continuous flow. By-products are frequently formed, and some of these products can be highly explosive. Most nitration reactions are carried out at low temperatures, primarily for safety reasons. Sometimes an aliphatic compound is used in the nitration reaction. Nonetheless, aromatic nitration is more commercially important.
Mechanism of electrophilic substitution reaction
An electrophilic substitution reaction is a chemical reaction in which a compound’s functional group is replaced by an electrophile. Typically, the displaced functional group is a hydrogen atom. Electrophilic substitution reactions are typically carried out in three steps, which are as follows.
- The emergence of an electrophile
- A carbocation’s formation (which is an intermediate)
- The extraction of a proton from an intermediate
Organic compounds undergo two types of electrophilic substitution reactions: electrophilic aromatic substitution reactions and electrophilic aliphatic substitution reactions. Below is an illustration describing the electrophilic substitution of a hydrogen atom (from a benzene molecule) with a chlorine atom.
Three steps are involved in the electrophilic substitution reaction mechanism.
Step 1: Create an Electrophile
Anhydrous aluminium chloride is a very useful Lewis acid for producing electrophiles from the chlorination, alkylation, and acylation of an aromatic ring. The resulting electrophiles (from the combination of anhydrous aluminium chloride and the attacking reagent) are Cl+, R+, and RC+O.
Step 2: Carbocation Formation
The electrophile attacks the aromatic ring, resulting in the formation of a sigma complex or an arenium ion. One of the carbons in this arenium ion has undergone sp3 hybridization. In a resonance structure, this arenium ion finds stability. Because electron delocalization terminates at the sp3 hybridised carbon, the sigma complex or arenium ion loses its aromatic character.
An atom attached to an aromatic ring is replaced with an electrophile in electrophilic aromatic substitution reactions. Aromatic nitrations, aromatic sulphonation, and Friedel-Crafts reactions are examples of such reactions. It is important to note that in electrophilic aromatic substitutions, the aromaticity of the aromatic compound is preserved. As a result, aromatic rings and iodine, bromine, or chlorine can be used in these reactions to produce aryl halides.
Electrophilic addition reaction of alkenes
As we prepare to delve deeper into the addition of alkenes via an electrophilic reaction, it is critical to understand the two other ways this process occurs. They are ozonolysis and oxidation reactions, respectively (particularly with alkenes). Hydrogen chloride and hydrogen bromide are two common examples of electrophilic addition reactions with hydrogen halides. It can also happen through the Free Radical Mechanism procedure. In the free radical group of compounds, free radical mechanism reactions follow the hierarchy of Chain Initiation, Chain Propagation, and Chain Termination (reaction with stable molecules). The widely accepted hydrogen halide order is HI > HBr > HCl.
When compared to non-symmetrical alkenes like propene (double bonds have a varied count of ligands), symmetrical alkenes like ethene are easily predictable in their end products. This phenomenon is also known as Markownikoff’s or Markovnikov’s rule.
As a result, these are the various chemical reactions that can be formed when alkenes are used, particularly in electrophilic addition reactions. When a group of elements undergoes various reactions at different states, each group of elements will react uniquely. As we saw with oxidation, it may vary in other states with different chemicals at different temperatures. As a result, before performing any reaction, one should understand the concept and significance of that reaction.
Also read: Preparation and Properties of Sodium hydroxide
FAQs
Is hydrogenation considered an electrophilic addition?
Another alkene reaction worth mentioning is hydrogenation, which is unrelated to the mechanism of electrophilic addition. Hydrogenation is the addition of molecular hydrogen (H2) to an alkene double bond. It converts an alkene into an alkane.
What is the distinction between electrophilic and nucleophilic addition?
An electron pair is embraced by the group being introduced in electrophilic addition, whereas an electron pair is donated by the added group in nucleophilic addition.
What Are the Different Kinds of Electrophilic Substitution Reactions?
Electrophilic aliphatic substitution and electrophilic aromatic substitution are the two main types of electrophilic substitutions.









