Table of Contents
Introduction
Chemistry is the science of how atoms and molecules interact with one another at the atomic level. The mole idea, which we will discuss here, bridges this gap by linking the mass of a single atom or molecule in AMU(Atomic Mass Unit) to the mass of a huge collection of similar molecules in grams.
As you know, the mass number is the sum of the number of protons and neutrons in an atom’s nucleus. The mass number is an integer that is roughly equal to the atomic mass’s numerical value. Despite the fact that the mass number is unitless, it is allocated units known as atomic mass units (AMU).
Since a molecule or polyatomic ion is an assembly of atoms whose identities are specified in its molecular or ionic formula, we can compute the average atomic mass of any molecule or polyatomic ion by summing the masses of the constituent atoms. Because the mass of electrons is so little that it is unimportant in most computations, the average mass of a monatomic ion is the same as the average mass of an atom of the element.
This isn’t much help in the laboratory for the average chemist, who just has a balance to weigh out compounds but has to know how many atoms or molecules are present. Clearly, something smart is required, but first, let us go through how molecular masses are determined.
Overview
As we have seen before, one substance’s identity is defined not only by the types of atoms or ions it contains but also by the quantity of each type of atom or ion. Water and hydrogen peroxide, for example, are similar in that their molecules are made up of hydrogen and oxygen atoms. Although, because a hydrogen peroxide molecule has two oxygen atoms, as opposed to a water molecule, the two compounds have quite different properties.
We now have powerful devices that allow us to directly measure these distinguishing microscopic qualities; nevertheless, the same traits were initially derived via measuring macroscopic properties (the weights and volumes of bulk amounts of matter) with quite primitive techniques (balances and volumetric glassware). This innovative method required the development of a new measure of substance amount, the mole, which is still used in modern chemical studies.
A mole is a unit of measurement that is similar to conventional units such as pair, dozen, bulk, and so on. It gives a precise count of the number of atoms or molecules in a large sample of materials. A mole is defined as the amount of substance containing the same number of discrete entities (atoms, molecules, ions, and so on) as the number of atoms in a 12 g sample of pure C.
The word “mole” has a Latin sense of “large mass” or “bulk,” which is compatible with its use as the name for this unit. The mole connects an easily measurable macroscopic feature, bulk mass, to an immensely significant fundamental property, number of atoms, molecules, and so forth.
Mole Concept and Molar Mass (Molecular Mass)
The mole concept is a simple way to indicate the amount of a substance. Any measurement is divided into two parts: the numerical magnitude and the units in which the magnitude is expressed. For example, if the mass of a ball is 2 kilograms, the magnitude is ‘2’ and the unit is ‘kilogram.’
When working with particles at the atomic (or molecular) level, it is recognized that even one gram of a pure element has a large number of atoms. This basically focuses on the ‘mole,’ which is a count of a very large number of particles.
The count of units forming a mole has been calculated as 6.02214179 1023. This is called as Avogadro’s number (NA) or Avogadro constant. It is perfectly reported using an explicit unit called per mole.
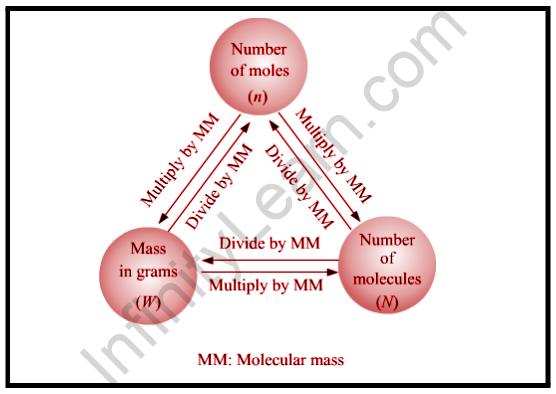
We can say that one substance’s molecular mass is the sum of the average masses of the atoms in one molecule of that substance. It is computed by multiplying the atomic masses of the elements in the material by their subscripts (written or inferred) in the molecular formula. Because atomic mass units are atomic mass units, molecular mass units are likewise atomic mass units.
Some facts about Mole and it’s concept:
The molar mass of a substance is expressed as the amount in grams of one mole of that substance. One mole of isotopically pure carbon-12 weighs 12 g. The molar mass of an element is the mass of 1 mol of atoms of that element; the molar mass of a covalent molecular compound is the mass of 1 mol of molecules of that compound; and the molar mass of an ionic compound is the mass of 1 mole of formula units. That is, a substance’s molar mass is the mass (in grams per mole) of 6.02214179 1023 atoms, molecules, or formula units. In each case, the number of grams in 1 mol is the same as the number of atomic mass units used to represent the atomic mass, molecular mass, or formula mass.
Because carbon occurs as a mixture of carbon-12, carbon-13, and carbon-14, the molar mass of naturally occurring carbon differs from that of carbon-12 and is not an integer.
Although atomic mass and molar mass are quantitatively similar, keep in mind that they are considerably different in terms of scale, as evidenced by the magnitudes of their respective units (AMU versus g). Consider a little drop of water weighing roughly 0.03 g to get a sense of the mole’s size (see Figure 3). The number of molecules in a single droplet of water is approximately 100 billion times more than the total number of humans on the planet.
The connections between formula mass, mole, and Avogadro’s number can be used to calculate a variety of numbers that define the composition of substances and compounds. For instance, if we know the mass and chemical makeup of a substance, one can compute the number of moles and atoms or molecules in the sample. Similarly, if we know the number of moles in a substance, we can deduce the number of atoms or molecules and determine the mass of the substance.
Also read: Free, Forced and Damped Oscillations
The information about the mole concept and molar mass from various physics-related articles is available here. Mole concept and molar mass are important topics in physics. Students who want to flourish in physics need to be well known about atoms, elements, and molecules to get deep knowledge about it to do well on their exams. The definitions and brief explanations are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional physics help.
FAQs:
Question 1: Why do we need the Mole concept?
Answer 1: It enables a scientist to weigh two substances, say iron and sulfur, in order to achieve an equal quantity of iron and sulfur atoms. A mole of a substance is defined as a material mass containing exactly 12,000 g of 12C and the same number of fundamental units as atoms.
Question 2: What is the use of the mole concept?
Answer 2: One mole is a unit of chemical quantity. It links the atom to the macroscopic quantities of material we work on within the laboratory. It enables a scientist to weigh two substances, say iron and sulfur, in order to achieve an equal quantity of iron and sulfur atoms.
Question 3: How many moles are in a mole?
Answer 3: A mole, usually mol, is a SI unit that counts the number of particles in a given substance. 1 mole is equivalent to 6.02214179 or other basic units such as molecules.






