Table of Contents
Monosaccharides also known as simple sugars, are the most basic units (monomers) of carbohydrates. The general formula is CnH2nOn, or [Cn(H2O)n] or CH2On, though not all molecules fitting this formula are carbohydrates (e.g., acetic acid). They are typically colourless, water-soluble solids. Despite their name, only a few monosaccharides have a sweet flavour (sugars). Monosaccharides include glucose (dextrose), fructose (levulose), and galactose. Disaccharides (such as sucrose and lactose) and polysaccharides are built on top of monosaccharides (such as cellulose and starch). Except for those at the end of the chain, every carbon atom that supports a hydroxyl group is chiral. This results in a number of isomeric forms, all of which have the same chemical formula. Galactose and glucose, for example, are both aldohexoses with distinct physical and chemical properties. The monosaccharide glucose is important in metabolism because it extracts chemical energy through glycolysis and the citric acid cycle to provide energy to living organisms. Other monosaccharides can be converted to glucose in living organisms. Glucose is a hexose that is used as an energy source and in the synthesis of starch, glycogen, and cellulose.
Pentose sugars are ribose and deoxyribose (in RNA and DNA, respectively). Ketones, mannoheptulose, and sedoheptulose are examples of heptoses. Monosaccharides with eight or more carbons are uncommon because they are highly unstable. If a monosaccharide has more than four carbons, it exists as a ring in aqueous solutions.
The most basic type are monosaccharides, which are simple sugars. This means that hydrolysis cannot break them down any further into simpler sugars. Monosaccharides, on the other hand, can combine to form more complex types. Glycosidic bonds (also called glycosidic linkages) are the covalent bonds that join monosaccharides. A disaccharide is a carbohydrate composed of two simple sugars, whereas oligosaccharides are composed of three to ten simple sugars, and polysaccharides are composed of a greater number of monosaccharide units.
Monosaccharides are typically colourless, crystalline solids with a sweet taste. They can be dissolved in water and occur as syrups or liquid sugar. Monosaccharides, like other carbohydrates, are organic compounds. They contain carbon that is covalently bound to other atoms, particularly Carbon-Carbon (C-C) and Carbon-Hydrogen (C-H) (C-H).
Overview
Poly-hydroxy-aldehydes or -ketones with an unbranched C-chain are monosaccharides. A carbohydrate is an organic compound with the formula (CH2O), where n is greater than three. Monosaccharides are the most basic carbohydrate kind. The majority of organisms generate and store energy by breaking down the monosaccharide glucose and harvesting the energy released. The number of carbon atoms and the functional group attached to this type of glucose is used to classify it. Aldose is a monosaccharide that contains aldehyde, and ketose is a monosaccharide that contains a ketone group.
All the monosaccharides have the formula as (CH2O) n. The two hydrogen atoms and one oxygen atom form a bond with the central carbon molecule. When oxygen bonds with hydrogen, a hydroxyl group is formed. Because carbon may create four bonds, several carbon molecules link together. One of the carbons in the chain will form a double bond with oxygen, forming a carbonyl group. If it is formed at the end of the chain, the monosaccharides are said to belong to the aldose family; if it is formed in the middle of the chain, the monosaccharides are said to belong to the ketose family.
Fructose is a straightforward ketonic monosaccharide. Monosaccharides are the basic units of carbohydrates that cannot be broken down further into simpler compounds. The functional group attached to the carbohydrate determines their classification.
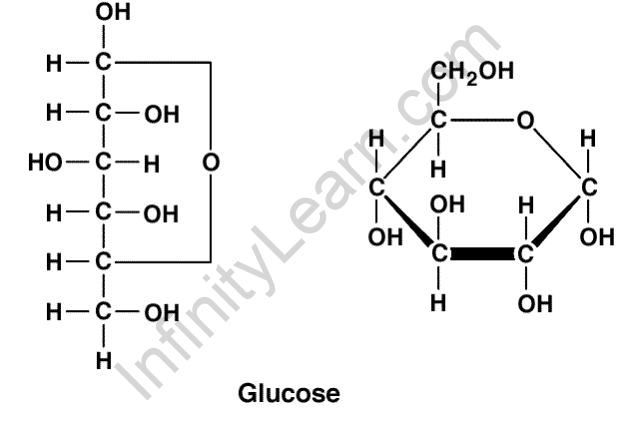
Glucose is a simple sugar with the molecular formula C6H12O6 that belongs to the carbohydrate group. There are six carbon atoms in it, as well as an aldehyde group. It’s called aldohexose as a result of this. It comes in two varieties: open-chain (acyclic) and ring (cyclic). The primary source of energy for living creatures is glucose. During the photosynthesis process, plants and algae use water, sunlight, and carbon dioxide to produce glucose. It can be found in fruits and honey in its natural state. Glucose is obtained in animals through the glycogenolysis process.
Monosaccharides examples
Glucose is a monosaccharide that occurs naturally and is found everywhere. It can bind to other monosaccharide units to form disaccharides such as maltose (two glucose molecules), lactose (two glucose and galactose molecules), and sucrose (i.e. glucose and fructose molecules). In plants and other photosynthetic organisms, glucose is one of the products of photosynthesis. Glucose molecules are stored in plants as sugar repeating units (e.g. starch). It is also a constituent of amylopectin and cellulose. As a result, it is abundant in fruits, plant juices, and many other plant organs. It is also an important metabolic intermediate in cellular respiration and a significant source of energy (via aerobic respiration or anaerobic respiration). It circulates in the blood of animals and is thus referred to as blood. In animals, an excess of glucose is stored as glycogen.
In terms of chemical structure, galactose is similar to glucose. The orientations of H and OH on carbon 4 are, however, switched. Galactose, unlike glucose, does not exist in a free state. It is commonly found as a component of complex biomolecules. For example, galactose and glucose combine to form lactose (milk sugar), which is a disaccharide. Lactose, the milk disaccharide, is composed of galactose linked to glucose via a -(1-4) glycosidic link. The enzymes lactase and -galactosidase catalyse the joining of galactose and glucose. The Leloir pathway is used for galactose catabolism (the conversion of glucose to glucose). One source of lactose in breast milk during human lactation is the de novo synthesis of galactose and glucose via hexoneogenesis. Galactose monomers link together to form galactan, a polysaccharide found in plants such as axlewood (Anogeissus latifolia) and acacia trees.
The sweetest naturally occurring carbohydrate is fructose. Honey, fruits, and sugar cane are all-natural sources of fructose. Because it has a reducing group (carbonyl) at carbon 2, it is a ketonic monosaccharide. This contrasts with glucose (an aldose), which has its carbonyl group at carbon 1. Fructose is found naturally in plants, particularly fruits and root vegetables. It can exist freely or it can be bonded to glucose to form sucrose.
Monosaccharide definition
Any of the basic compounds that serve as the building blocks of carbohydrates are referred to as monosaccharides or simple sugars. Monosaccharides have more than one hydroxyl group (-OH) and a carbonyl group (-C=O) at the terminal carbon atom known as an aldose or the second carbon atom known as a ketose. Polyhydroxy aldehydes and ketones are molecules with such structures.
The number of carbon atoms in a monosaccharide molecule determines its classification: There are two dioses.
- Trios have three members.
- four tetroses
- Five pentoses
- Six hexoses
- Heptoses number seven.
These various monosaccharides can be found in woody materials such as xylem or as arabinose from coniferous trees, as well as in our bodies as ribose, a component of ribonucleic acids (RNA) and several vitamins.
Types of monosaccharides
Monosaccharides are classified into two types based on the functional group they contain. As a result, if they contain an aldehyde group, they are referred to as “aldose.” And if they contain a keto group, we refer to them as “ketose.” There is also a classification based on the number of carbon atoms in each molecule.
Monosaccharides are classified as follows:
- Monosaccharides with neutral pH
- Osamines
- Uronic acids
- Acids sialic
Monosaccharides that are neutral: They include carbohydrates that have only an alcohol group in addition to their ketone and aldehyde groups. D-glucose, D-galactose, D-mannose, and D-xylose are a few examples. This type of monosaccharide also includes Deoxys, which are monosaccharides that have lost one or two oxygen atoms.
Osamines: They are made up of neutral monosaccharides. An amine group replaces the neutral monosaccharide hydroxyl (generally the one carried by carbon 2).
Uronic acid (Uronic acid): Uronic acids are created by converting the primary-alcohol group into a carboxylic group (and therefore maintaining the aldehyde group).
Acids sialic: Sialic acids are derived from neuraminic acid, which is composed of a molecule of pyruvic acid condensed with a molecule of D-monoamine.
FAQs
What are the main distinctions between glucose and fructose?
The primary distinctions between glucose and fructose are as follows: Fructose and glucose are both monosaccharide sugars that are simple to produce. When starch and sugar, whether sucrose or high-fructose corn syrup (HFCS), are digested, a large amount of glucose is produced.
What are the various types of glucose?
The d-isomer, d-glucose, also known as dextrose, is abundant in nature, whereas the l-isomer, l-glucose, is not. To obtain glucose, carbohydrates such as dairy sugar (lactose), plant sugar (sucrose), maltose, cellulose, glycogen, and others can be hydrolyzed.
What exactly are fructose and glucose?
Fructose and glucose are both monosaccharide simple sugars. Both starch and sugar, whether sucrose or high-fructose corn syrup (HCFS), produce a large amount of glucose when digested.



