Table of Contents
Polymers seem to be macromolecules composed of small units known as monomers. Polymers include a wide range of compounds that are extremely useful in our daily lives. These polymers can be classified in a variety of ways, including structure, chemical or physical properties, and so on. Polymers are classified into two categories: natural polymers and synthetic polymers. Natural polymers are polymer compounds that can be found in nature. Synthetic polymers are artificially produced polymer compounds. This is the primary distinction between natural and synthetic polymers.
Overview
Polyester fibres are often spun with natural fibres to create a cloth with a mix of properties. Cotton-polyester blends can be durable, wrinkle- and tear-resistant, and shrink-resistant. When compared to plant-derived fibres, synthetic fibres made of polyester have superior water, wind, and environmental resistance. They have lower fire resistance and can melt if ignited. Natural polymers were indeed polymer compounds that can be found in the environment. Polymer compounds make up the majority of chemical compounds found in biological systems. Polysaccharides, polyamides, and polynucleotides are the three main types of natural polymers. Humans create synthetic polymers by synthesising polymer compounds. Depending on the need, these polymer productions are carried out in laboratories or factories. Several chemical reactions are used to create these polymers. Polymers can thus be classified further based on the type of chemical reaction used.
Polyesters
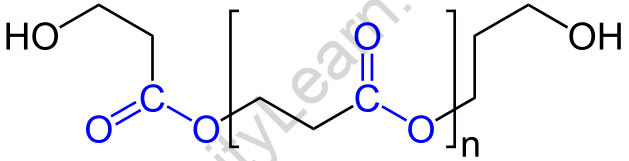
Polyester is a type of polymer that has an ester functional group in each repeat unit of its main chain. It most commonly refers to a type of material known as polyethene terephthalate (PET). Polyesters comprise both naturally occurring chemicals such as those found in plants and insects and synthetics such as polybutyrate. We can say that natural and a few synthetic polyesters are biodegradable, but the vast majority of synthetic polyesters are not. Synthetic polyesters are widely used in clothing.
Liquid crystalline polyesters were one of the first liquid crystal polymers to be used in industry. They are used for their mechanical and heat-resistance properties. These characteristics are also important in their use as an abradable seal in jet engines.
Natural polyesters may well have played an important role in the evolution of life. Under simple prebiotic conditions, long heterogeneous polyester chains and membranes structures are known to form easily in a one-pot reaction without a catalyst.
Natural and synthetic like polyesters
Polymers are basically divided into two types: synthetic and natural. Scientists and engineers create synthetic polymers out of petroleum oil. In fact, nylon, polyethylene, polyester, Teflon, and epoxy are examples of synthetic polymers. Natural polymers can be extracted from nature. They are frequently water-based. Additionally, silk, wool, DNA, cellulose, and proteins are typical examples of naturally occurring polymers.
Rubber could be found in nature and extracted as a latex (milky liquid) from various tree species. Natural rubber derived from tree latex is essentially a polymer composed of isoprene units with a trace of impurity. Man can also create (synthesise) rubber. A variety of monomers, including isoprene, can be polymerized to produce synthetic rubber.
Natural rubber is difficult to work with (it is sticky), and it has poor properties and durability (it rots). It is usually vulcanised, which is a process that involves heating the rubber in the presence of sulphur to improve its resilience, elasticity, and durability. Synthetic rubber has been favoured because different monomers can be mixed in different proportions to produce a wide range of physical, mechanical, and chemical properties. Monomers can be manufactured pure, and the addition of impurities or additives can be controlled by design to provide optimal properties.
Vulcanization, as well known as curing, is a chemical method used in the rubber industry in which individual polyisoprene chains are chemically linked to other polyisoprene chains. The actual chemical cross-linking is usually done with sulphur, but other technologies can be used as well. Vulcanization is said to be an irreversible process. Rubber molecules, which are normally soft and springy, become locked together, resulting in a harder material with greater durability and chemical resistance. Vulcanization transforms the material’s surface from a very sticky to a smooth, soft surface that does not adhere to metal or plastic substrates.
Pectin is a polymer with a long chain made up of pectic acid and pectinic acid molecules. Pectin is classified as a polysaccharide because these acids are sugars. It is made from citrus peels and apple remnants. Pectin is the material that holds plant cells together in the plant/fruit.
The pectin chains create a network because some of the segments of the pectin chains crystallise together to form a three-dimensional network that holds water, sugar, and other materials. Physical or chemical changes that tend to decrease the solubility of the pectin and favour the formation of small localised crystals cause the formation of a gel. Temperature is the most important factor influencing pectin’s tendency to gel.
Whenever a hot solution containing pectin is cooled, the molecules move less and have a greater tendency to combine into a gel network. Because of this property, pectin is an excellent thickener for a variety of foods, including jellies and jams. Pectin forms a firm gel in the presence of enough sugar in the mixture.
Synthetic fibre
Synthetic fibres, as well known as synthetic fibres, are fibres created by humans through chemical synthesis, as opposed to natural fibres that are derived directly from living organisms, such as plants (such as cotton) or animal fur. They are the result of extensive scientific research to improve naturally occurring animal and plant fibres. Synthetic fibres are generally made by extruding fibre-forming materials through spinnerets, resulting in a ‘different’ fibre. These are referred to as synthetic or artificial fibres. Synthetic fibres are made through the polymerization process, which involves combining monomers to form a long chain or polymer.
Synthetic fibres were indeed man-made fibres, with the majority of them made from petroleum raw materials known as petrochemicals. All fabrics are made from fibres, which can be obtained from natural or artificial sources. These are build-ups of a small unit or polymer that is made up of many repeating units known as monomers. Generally, nylon, acrylics, polyurethane, and polypropylene are among them. Every year, millions of tonnes of these fibres are produced around the world.
Synthetic fibres examples
Rayon: It is a semisynthetic material composed of wood pulp (cellulose), carbon disulphide, and sodium hydroxide. It’s being used to imitate natural fibres such as cotton and silk. There are also different subtypes of rayon.
Nylon: It is one of the most popular synthetic fibres and is entirely derived from chemical processes. Nylon is more hydrophilic than the polymers discussed above due to its amide backbone. Notice how your nylon clothing absorbs water; this is because nylon, unlike the pure hydrocarbon polymers that make up the majority of plastics, can form hydrogen bonds with water.
Polyester: Some other common man-made fibre, it is produced chemically from plant proteins and is widely used in the production of plastic bottles. The top features are its high strength and longer shelf life.
In addition, a few other types of synthetic fibres are used for non-textile applications. Those are dacron, lyocell, modal, PAN, asbestos, spandex, and polyurethane. Some of these are combined with natural fibres to create advanced fabrics that combine both of their properties. A stretchable fabric used for shirting and other clothing materials is an example of this method. It not only improves the appearance and feels, but it also increases the quality.
Natural fibre
The natural fibre is said to be a certain hairlike raw material obtained directly from an animal, vegetable, or mineral source and convertible into nonwoven fabrics such as felt or paper, or, after spinning into yarns, woven cloth. The natural fibre is further defined as an agglomeration of cells with a diameter that is insignificant in comparison to its length. Although nature is abundant in fibrous materials, particularly cellulosic types such as cotton, wool, grains, and straw, only a small number of them can be used for textiles or other industrial purposes. Aside from economic considerations, the commercial usefulness of a fibre is determined by properties such as length, strength, pliability, elasticity, abrasion resistance, absorbency, and various surface properties. The majority of textile fibres are thin, flexible, and relatively strong. They are elastic in the sense that when tension is applied to them, they stretch and then partially or completely return to their original length when the tension is released.
FAQs
Is polyester better than cotton?
Polyester is more powerful than cotton and has a greater ability to stretch. Fibre strength can range from 2.5 to 9.5 grammes per denier. Buyers who are concerned about the environment are opposed to the use of polyester.
Is polyester good for skin?
Polyester as well as polyester blends: Polyester, like nylon, is water repellent, allowing perspiration to pass between our clothes and your skin, causing the garment to stick to your body.
How do polyesters form?
A polyester is created through the reaction of an acid with two -COOH groups and alcohol with two -OH groups.









