Table of Contents
An oxyacid, otherwise called an oxoacid or ternary corrosive, is corrosive containing oxygen. It is a compound containing hydrogen, oxygen, as well as a minimum of only one component, with somewhere around one hydrogen iota bound to oxygen and equipped for separating to make the corrosive’s H+ cation and anion. All acids, as per Lavoisier’s unique speculation, contain oxygen, which was so-called after the Greek (oxys: corrosive, sharp) and the root – v (qualities – maker). After it was initiated that a few acids, including hydrochloric corrosive, needed oxygen, acids were isolated into oxo-acids and every one of these new hydro acids.
A Brief Outline
Likewise, with paired nonmetal hydrides, the acidic hydrogen is attached to an oxygen particle in all oxyacids, henceforth bond strength (length) isn’t a thought. Oxyacid is not entirely set in stone by the electronegativity of the center component (X) and the number of O molecules. Corrosive strength develops as the number of oxygens associated with the center component X increments. Corrosive strength develops as the electronegativity of X increments with a similar number of oxygens around E. Oxyacids are by and large less steady than salts of their deprotonated structures, the oxyanions, and a significant number of them just exist in fact as theoretical species or just exist in arrangement and can’t be disconnected in an unadulterated state.
Important concepts
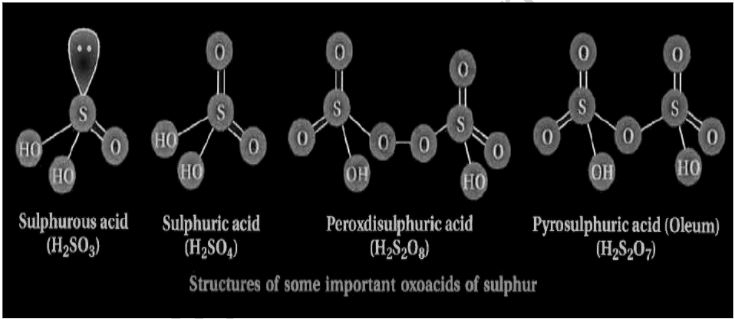
Oxoacids of sulfur structure
Oxoacids are acids that have oxygen in them. Sulfur is perceived to make an assortment of oxoacids, including H2SO4, H2SO3, and others. Whenever sulfur is composed of oxygen in oxoacids, it takes on a tetrahedral structure. Sulfur oxoacids as a rule have no less than one S=O bond and an S-OH bond. Notwithstanding S=S and S-OH, there exist terminal peroxide gatherings, terminal S=S, terminal and crossing over oxygen molecules, and chains of (- S-) n.
Sulphuric acid (H2SO4)
Sulphuric corrosive is among the most frequently utilized sulfur oxoacids. It’s a sort of diprotic corrosive (it ionizes to give two protons). One particle of sulfur is associated with two hydroxyl bunches in sulphuric corrosive, while the leftover two oxygen molecule’s structure pie associations with the sulfur iota. Thus, sulfuric corrosive has a tetrahedral shape.
Sulfurous acid (H2SO3)
Sulphuric corrosive is a diprotic corrosive, meaning it ionizes two photons simultaneously. One sulfur particle is associated with two hydroxyl bunches in sulfurous corrosive, and one oxygen iota shapes a pie association with the sulfur molecule. Sulfur dioxide is broken down in the water and makes it.
Peroxodisulphuric acid (H2S2O8)
Sulfur in the oxidation state is available in Peroxodisulphuric corrosive. Thus, it is a strong oxidizer and exceptionally burnable in nature. Marshall’s corrosive is the normal name for it. It has one peroxide bunch, which goes about as a connection between the two Sulfur particles. Other than the peroxide bunch, each Sulfur molecule is coupled to one hydroxyl bunch (S-OH bond) in addition to two oxygen particles (S=O bond).
Pyrosulphuric Acid (H2S2O7)
Oleum is another name for pyrosulphuric acid. It has a molality of 178.13 g/mol. It’s a white, crystalline solid with a melting point of 36°C that’s an anhydride of sulphuric acid. Excess Sulphur trioxide can be made by reacting it with sulphuric acid. It interacts with bases to generate pyrosulphates, which are salts. It’s used in the production of explosives and dyes. It’s also utilized in the refinement of petroleum.
The oxidation state of oxoacids of sulfur:
In sulfur, the maximum oxidation state is +6. In general, we find that sulfur oxo-acids exhibit oxidation states of +4 and +6. In oxo acids, sulfur does not have a negative oxidation state.
Physical Properties
- Pure sulphuric acid has a specific gravity of 1.84at288K and is a colourless, thick, oily liquid.
- Sulphuric acid is water-soluble. When sulfuric acid is combined with water, it produces a lot of heat and a lot of volume loss. The heat produced while diluting concentrated sulfuric acid has the possibility to trigger an accident. To avoid mishaps, always dilute concentrated acid with water rather than dilute acid with water.
Chemical Properties:
- When water-free sulphuric acid is cooked, sulfur trioxide and water are produced.
- Characteristics of Acidity It is a dibasic acid that changes the colour of blue litmus to red. It creates two series of salts when it interacts with alkali.
There are various explanations behind the presence of oxoacids:
(1) They might gather to shape oligomers or get dried out totally to make anhydrite,
(2) they might be imbalanced to one synthetic of more noteworthy oxidation state and one more of lower oxidation state,
(3) they might exist absolutely as another, more steady tautomeric structure, phosphonic corrosive.
Significance of oxoacids of Sulphur in NEET exam
Science is a subject that demands a solid comprehension of focuses to complete board tests and other relentless tests. Preparing for a subject without adequate audit materials and plans, of course, can testing. Subsequently, suggesting science answers or practicing from these materials will help students in getting extraordinary scores on the NEET test. The models overall and requests in Infinity Learn to rely upon the CBSE outline, and there is a potential that vague requests will appear in the test. The critical target of the plans is for understudies to have the choice to self-analyze their weaknesses and progress around there.
FAQ’s
Sulfur can be found in its normal state as well as in metal sulfide minerals. It very well may be found in its regular state around volcanoes and underground aquifers. Sulfur is the 10th most pervasive component on earth, and it very well may be found in shooting stars, the sea, the world's outside, the environment, and practically all plant and creature life.
As a strong corrosive and an oxidizing specialist, nitric corrosive is generally used in research facilities and the substance business. The corrosive is generally utilized in the development of explosives, colours, polymers, and drugs. Manures containing nitrates are very significant.
Sulfur oxoacids are chemical substances that contain sulfur, oxygen, and hydrogen. The most well-known and commonly used industrially is sulfuric acid. Where would you be able to find down sulfur?
What are the uses of oxyacids?
What are Sulphur Oxoacids, and what do they do?








