Table of Contents
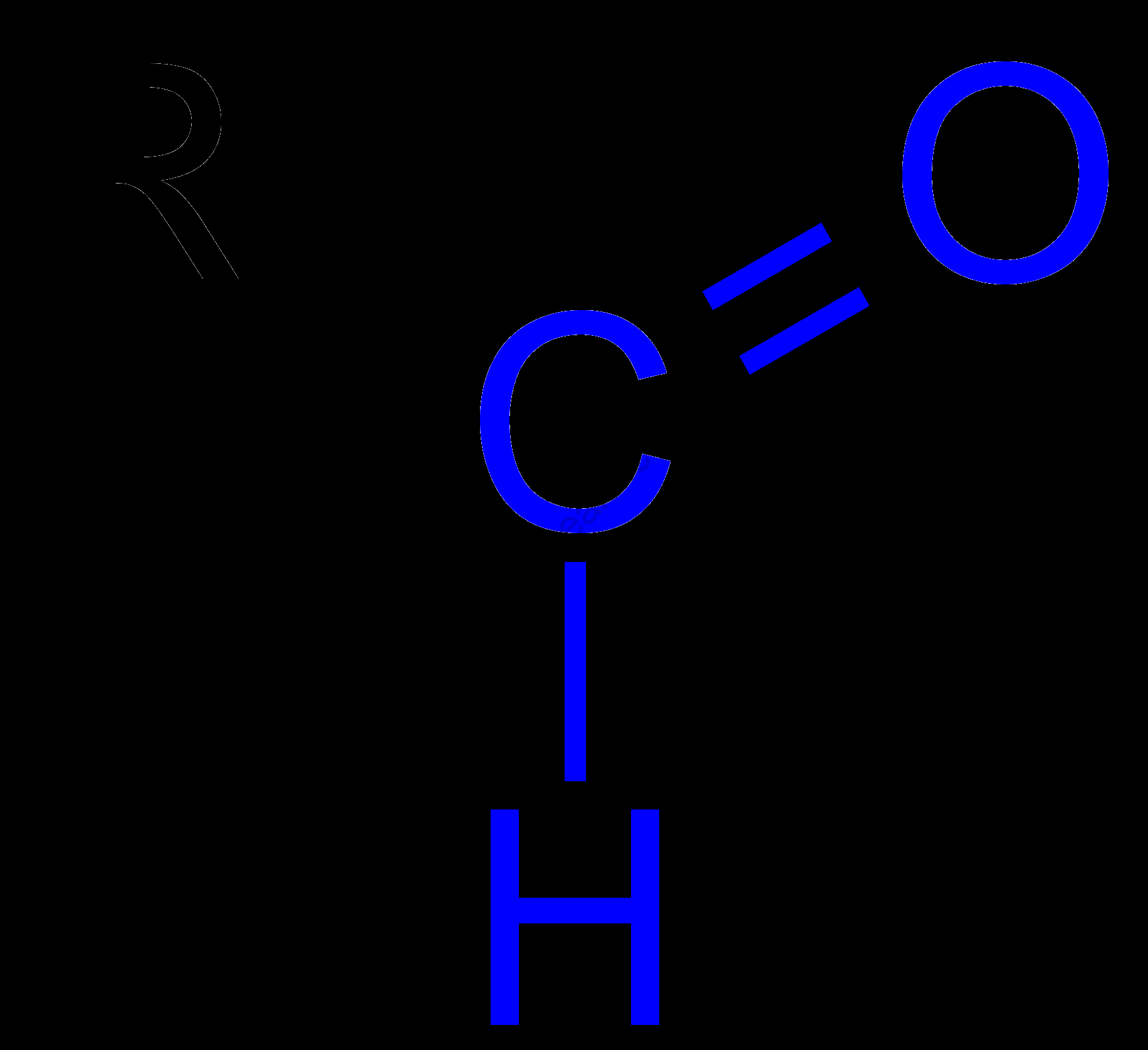
Aldehydes are members of a class of organic chemical composites represented by the general structural formula R-CHO. R may be hydrogen or a hydrocarbon revolutionary – substituted or unsubstituted.
Numerous aldehydes are unpredictable, ignitable liquids which at normal room temperature form vapour in explosive attention. Fire and explosion preventives must be most rigorous in the case of the lower members of the aldehyde family and safeguards with respect to irritant properties must also be most expansive for the lower members and for those with an unsaturated or substituted chain.
Uses of Aldehydes
- Formaldehyde is used as a detergent and as a preservative for natural specimens.
- Aldehyde is used for silvering glasses.
- Formaldehyde is used for the product of a variety of plastics and resins.
- Benzaldehyde is used in perfumery and in the dye (colour) industry.
Aldehydes and Ketones Physical properties
1. Physical State
Methanal is a pungent-smelling gas. Ethanol is an unpredictable liquid. Other aldehydes and ketones continuing up to eleven carbon electrons are tintless liquids while still advanced members are solids.
2. Smell (odour)
Except for the lower carbon aldehydes, which have unwelcome odours, all other aldehydes and ketones generally have an affable smell. As the size of the aldehyde and ketone patch increases, the odour becomes lower-pungent and more ambrosial. In fact, numerous naturally occurring aldehydes and ketones have been used in the blending of scents and spicing agents.
3. Solubility
Aldehydes and ketones up to four carbon electrons are miscible with water. This is due to the presence of hydrogen bond association between the polar carbonyl group and water motes as shown below
Still, the solubility of aldehydes and ketones in water decreases fleetly by adding the length of the alkyl chain ( carbon chain). As a result, the advanced members with further than four carbon electrons are virtually undoable in water. All aldehydes and ketones are answerable in organic detergents (like dissolves like) similar to benzene, ether, chloroform, and alcohol.
4. Boiling Point
The boiling points of aldehydes and ketones are more advanced than those of non-polar composites (hydrocarbons) or weakly polar composites of similar molecular millions. Still, their boiling point is lower than those of corresponding alcohols or carboxylic acids. This is because aldehydes and ketones are polar composites having sufficient intermolecular (between the motes) dipole-dipole relations between the contrary ends of carbonyl dipoles.
Chemical properties of Aldehydes and Ketones
The chemical properties of aldehydes and ketones are due to the polar carbonyl group present in their motes.
1. The reaction of aldehydes and ketones With Hydrogen Cyanide
Both aldehydes and ketones reply with hydrogen cyanide to form a fresh product known as cyanohydrins. The Reaction of aldehydes and ketones is carried out in the presence of an acid catalyst similar to aluminium chloride at a high temperature.
2. The reaction of ketones and aldehydes With Sodium Bisulphite
Both aldehydes and ketones form liquid addition composites called bisulfite adducts when treated with an impregnated result of sodium bisulphite. The result is boiled to drive off the redundant bisulphite and the product is also formed.
3. The reaction of aldehydes and ketones With Grignard Reagents
Aldehydes and ketones reply with a Grignard reagent to form fresh products. When the fresh product is hydrolyzed by water, it gives alcohol. For illustration, ethanol reacts with redundant Grignard reagent to give a fresh product called diethyl ether. Hydrolysis of this fresh product gives ethanol.
4. The reaction of aldehydes and ketones With Alcohols
Aldehydes reply with alcohols in the presence of dry HCl gas to give gem-dialkoxy composites. These composites are called acetals.
Ketone chemical structure
Ketones are organic composites that have the functional group C =O and the structure R- (C =O )-R’.
These carbonyl composites have carbon-containing substituents on both sides of the carbon-oxygen double bond. The carbonyl carbon of the ketone group is sp2 hybridized. The structure of ketones is often known as a trigonal planar centred around the carbonyl carbon. The bond angles of this structure are approximate 1200. Since the carbon-oxygen bond makes the carbonyl group polar (oxygen is further electron-withdrawing than carbon), ketones tend to be nucleophilic at the oxygen snippet and electrophilic at the carbon snippet.
Ketones are mass-produced industrially for their use as detergents, medicinals, and as precursors for polymers.
Some of the important ketones are methyl ethyl ketone (usually known as butanone), acetone and cyclohexanone.
Preparation of Ketones
Acid chlorides in the Reaction of aldehydes and ketones with dialkyl cadmium produce ketones.
Dialkyl cadmium is often prepared with the help of Grignard reagents.
2R-Mg-X CdCl2 → R2Cd + 2 Mg (X) Cl
2RCOCl R2Cd → 2R-CO-R + CdCl2
This method is useful in a way that the mixed ketones can be prepared very accessibly.
Uses of Ketones
- Propanone is used to make polymers for illustration perspex.
- Ketones are used as detergents and as a starting material for the conflation of numerous organic composites.
- Acetone and ethyl methyl ketone is substantially used as artificial detergents.
FAQs
What are the physical properties of aldehydes?
The carbonyl group’s opposition especially affects the physical properties of melting point and boiling point, solubility, and moment dipole. Hydrocarbons, composites that correspond only to the hydrogen and carbon rudiments, are basically nonpolar and thus have low melting and boiling points.
Why are aldehydes used in scents?
Aldehydes are the result of partial oxidation and frequently decide the moniker given to them from the name of the acid it forms. They're used to make synthetic resins and to produce colourings, flavourings, scents, and other chemicals. Others are used as preservatives and as detergents.
What are natural aldehydes?
Aldehydes are a family of reactive, organic composites that can be produced in natural products similar to the cinnamon dinghy (cinnamaldehyde) and vanilla bean (vanillin) as well as in laboratories.
Are ketones and aldehydes carboxylic acid derivatives?
The difference between carboxylic acid derivations and aldehydes and ketones is that there's a group containing a negatively charged heteroatom ( generally oxygen, nitrogen or sulfur), which is directly connected to the carbonyl carbon snippet. You can suppose carboxylic acid derivations are bilateral.









