Table of Contents
Introduction:
Defects that occur only at or near a single lattice point are referred to as point defects. They are not extended in any dimension of space. In most cases, strict limits on how small a point defect can be are not explicitly defined. However, these flaws are usually limited to a few extra or missing atoms. Larger defects in an ordered structure are commonly referred to as dislocation loops.
Many point defects, particularly in ionic crystals, are referred to as centres for historical reasons: for example, a vacancy in many ionic solids is referred to as a luminescence centre, a color center, or an F-center. Ionic transport through crystals is enabled by these dislocations, resulting in electrochemical reactions. Kröger–Vink notation is commonly used to specify these.
Vacancy defects are lattice sites that, in a perfect crystal, would be occupied but are not. If a neighboring atom moves to occupy the vacant site, the vacancy moves in the opposite direction to the site that the moving atom used to occupy. The surrounding crystal structure’s stability ensures that the neighboring atoms do not simply collapse around the vacancy. In some materials, neighboring atoms actually move away from a vacancy due to attraction from atoms in the vicinity. A point effect is a localized disruption in the regularity of a lattice.
The defect could be one or two atomic diameters in size, similar to a point. It only stretches a few diameters beyond the lattice position. A Schottky defect is a vacancy (or pair of vacancies in an ionic solid).
Overview
Point defects describe the imperfections of solids as well as the different types of point defects. Many small crystals are joined together to form crystalline solids. Following the crystallisation process, various types of defects are discovered in crystals. When the crystallisation process occurs at a rapid rate, point defects are accounted for. These flaws are caused primarily by variations in the arrangement of constituent particles. A point defect occurs when the ideal arrangement of solids in a crystalline solid is distorted around a point/atom. Line defects, point defects, volume defects, and surface defects are the four types of defects found in crystalline solids. Historically, crystal point defects were first observed in ionic crystals rather than metal crystals.
The three types of point defects are Frenkel, Schottky, and impurity. The Frenkel defect is caused by a single ion being displaced from its normal lattice point and shifting to a nearby interstice, or space between atoms in the lattice. Two ions of opposite signs leave the lattice in the Schottky defect. Impurity defects are foreign atoms that replace some of the atoms that make up the solid or squeeze into the interstices; they play a role in the electrical behaviour of semiconductors, which are materials used in computer chips and other electronic devices.
Line defects, also known as dislocations, are lines along which entire rows of atoms in a solid are arranged incorrectly. The resulting spacing irregularity is most pronounced along a line known as the line of dislocation. Line flaws can either weaken or strengthen a solid. Surface defects can form at the interface of two grains, or small crystals, within a larger crystal. Rows of atoms in two grains may run in slightly different directions, resulting in a mismatch across the grain boundary. Because the atoms on the surface adjust their positions to compensate for the absence of neighbouring atoms outside the surface, the actual external surface of a crystal is also a surface defect.
Types of Point Defects
Point defects are further classified as follows:
- Stoichiometric Defects – Stoichiometric compounds are those that follow the laws of definite proportions, constant composition, and mass conservation. Stoichiometric defects are defects in crystals that do not affect the stoichiometry of the compound or crystal.
- Vacancy Defect – A vacancy defect is a point defect that occurs when an atom is removed from its original lattice site. It causes the substance’s density to decrease. The number of vacancy defects varies with crystal temperature. It happens as a result of poor packing during crystallisation.
- Interstitial Defect – An interstitial defect is a point defect that occurs when an atom moves to the interstitial position of the lattice structure. The atom can be of the same crystal or of a different crystal or material. If the atoms are from the same crystal, the defect is known as a self-interstitial defect.
- Schottky Defect – A Schottky defect is a type of point defect that can be found in a variety of crystals. This defect, named after a German physicist, Walter H. Schottky, is common in ionic crystals where the cation and anion sizes are similar. Schottky defects are typically formed when an ionic compound crystal is heated. Heat causes the temperature to rise, and thus the thermal vibration within the crystal increase. As a result, gaps appear in the crystal pattern.
- Frenkel Defect – A Frenkel defect is a point crystallographic defect that is commonly found in ionic compounds. It is named after Soviet physicist Yakov Frenkel and differs from the Schottky defect in its occurrence and characteristics. The Frenkel defect is most common in ionic compounds with different sized ions.
- Non-Stoichiometric Defects – Stoichiometric defects are crystal defects that disrupt the stoichiometry of the compound or crystal. Non – Stoichiometric defects are classified into two types:
- Metal Excess Defects – As the name implies, metal ions occur in excess in the crystal lattice in this defect.
It can happen in one of two ways: Anionic Vacancy – Anion disappears from its corresponding lattice site, leaving a vacancy.
An electron fills this vacancy to keep the overall electric charge zero or neutral.
It’s known as the F – centre.
Schottky defect and frenkel defect
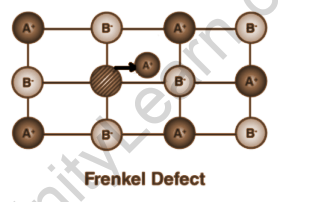
Frenkel defect
In general, the smaller ion (cation) moves out of its place and occupies an intermolecular space in ionic solids. In this case, the original position suffers from a vacancy defect, while the new position suffers from an interstitial defect.
- It is also referred to as a dislocation defect.
- A substance’s density remains constant.
- It occurs when anions and cations have a large size difference.
- ZnS and AgCl are two examples.
Schottky defect
Ionic Solids contain this type of vacancy defect. However, in ionic compounds, we must balance the electrical neutrality of the compound so that an equal number of anions and cations are absent. It makes the substance less dense. In this case, the sizes of cations and anions are nearly identical.
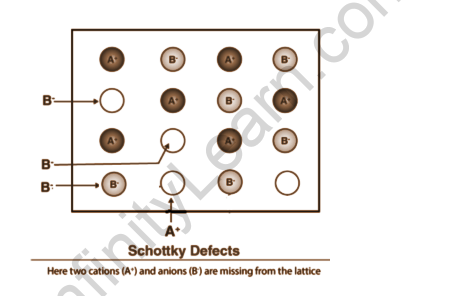
Example of Schottky defect
Ionic substances with almost identical cation and anion sizes exhibit the Schottky defect. NaCl, KCl, CsCl, and AgBr are a few examples. It should be noted that AgBr exhibits both Frenkel and Schottky defects. This type of defect is common in highly ionic compounds, highly coordinated compounds, and compounds with a small difference in the sizes of the cations and anions that make up the compound lattice. NaCl, KCl, KBr, CsCl, and AgBr are examples of salts that exhibit Schottky disorder. Schottky defects are important in oxides with Fluorite structures, such as CeO2, cubic ZrO2, UO2, ThO2, and PuO2, for engineering applications.
FAQ’s
Question 1: Which of the following are the most common types of crystal defects?
Answer 1: When an atom in a crystal misses a site or an impure atom occupies either a hole in the lattice between the atoms or a normal lattice site, this is referred to as a crystal defect. Surface defects, substitutional defects, line defects, point defects, interstitial defects, Frenkel and Schottky defects, and other types of Crystal defects are the most common.
Question 2: What exactly is the Frenkel and Schottky defect?
Answer 2: Both cation and anion leave the solid crystal in the Schottky defect. Only the smaller ion (cation) leaves its original lattice site in Frenkel defect, whereas the anion remains in its original lattice site. The atoms are permanently removed from the crystal.









