Table of Contents
One of most common form of natural carbohydrates is polysaccharide, also known as glycan. Polysaccharides can have either a branched or a linear molecular structure. Linear compounds, such as cellulose, frequently pack together to form a rigid structure; branched forms (e.g., gum arabic), on the other hand, are generally soluble in water and form pastes.
Polysaccharides are by far the most plentiful natural biopolymer and have unique chemical, physical, and biological properties. The backbone of these polymers is formed by monosaccharide building blocks and glycosidic linkages, which determine the diversity and complexity of the polysaccharides. These advancements continue to enable a growing number of exciting polysaccharide biomedical applications by academia and industry.
Overview
Polysaccharides are common biopolymers found throughout nature. These are polymers made up of monosaccharides linked together by glycosidic linkages. Polysaccharides are classified into several types. Polysaccharides have a molecular structure that can be linear or highly branched, and they can be made up of the same (homopolysaccharide) or different (heteropolysaccharide) monosaccharide units.
Physical and chemical properties are conferred by structural differences. Polysaccharides are biopolymers that are nontoxic and biodegradable. Polysaccharides can be found in abundance in algae. Algae are grown commercially in various parts of the world to produce hydrocolloids such as alginates and carrageenan.
Polysaccharides found in marine microalgae include cell wall polysaccharides, mucopolysaccharides, and storage polysaccharides. These polysaccharides are abundant in sea weeds, particularly red macro algae. It is very simple to collect seaweeds in their natural habitat.
These polysaccharides are used in a variety of commercial applications. They are widely used in food products as gelling agents, thickeners, stabilisers, and emulsifiers. They’re also used in pharmaceuticals, photography, and tertiary oil recovery.
Polysaccharides
Polysaccharides, also known as polycarbohydrates, are the most common carbohydrate found in food. They are polymeric long-chain carbohydrates made up of monosaccharide units linked together by glycosidic linkages. This carbohydrate can react with water (hydrolysis) with amylase enzymes acting as a catalyst, resulting in constituent sugars (monosaccharides, or oligosaccharides). They have a variety of structures ranging from linear to highly branched.
Polysaccharides are frequently quite heterogeneous, with minor variations in the repeating unit. These macromolecules can have properties that differ from their monosaccharide building blocks depending on their structure. They may be amorphous or even water-insoluble. Whenever all of the monosaccharides in a polysaccharide are of the same type.
The polysaccharide is referred to as a homopolysaccharide or homoglycan; however, when more than one type of monosaccharide is present, the polysaccharide is referred to as a heteropolysaccharide or heteroglycan.
Natural saccharides are mostly made up of simple carbohydrates known as monosaccharides, which have the general formula (CH2O)n, where n is three or more. Monosaccharides include glucose, fructose, and glyceraldehyde.
Polysaccharides, on the other hand, have the general formula Cx(H2O)y, where x is typically a large number between 200 and 2500. When the repeating units in the polymer backbone are six-carbon monosaccharides, as they are frequently, the general formula simplifies to (C6H10O5)n, where 40 ≤ n ≤ 3000.
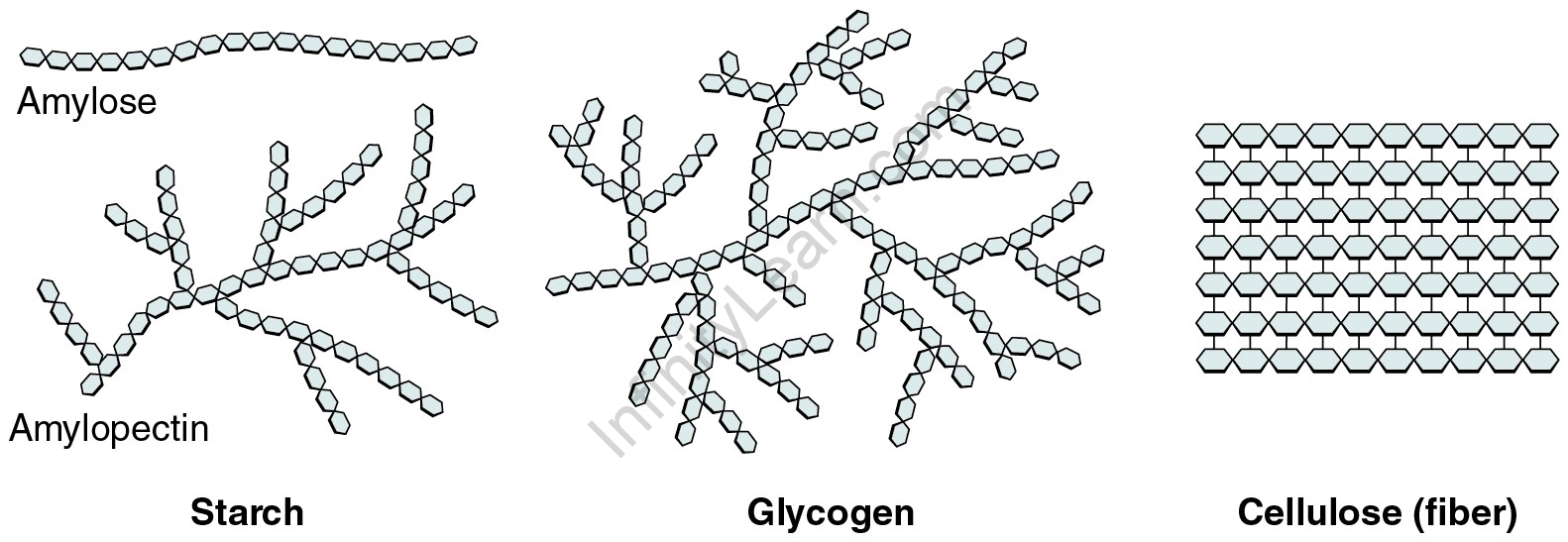
Polysaccharides have more than ten monosaccharide units, whereas oligosaccharides have three to ten monosaccharide units; however, the precise cut-off varies slightly depending on convention. Polysaccharides are a type of biological polymer that is widely used. In living organisms, their function is usually structural or storage-related. Starch (a polymer of glucose) is found in plants as a storage polysaccharide in the forms of amylose and branched amylopectin.
The structurally similar glucose polymer in animals is the more densely branched glycogen, also known as “animal starch.” Glycogen’s properties allow it to be metabolised more quickly, which is ideal for moving animals’ active lifestyles. They play an important role in bacterial multicellularity.
Storage polysaccharides such as starch, glycogen, and galactogen are examples, as are structural polysaccharides such as cellulose and chitin.
Polysaccharide examples: Polysaccharides (starch, cellulose, glycogen)
Storage polysaccharides:
Starch is really a glucose polymer with alpha-linkages that connect glucopyranose units. It is composed of a mixture of amylose (15–20%) and amylopectin (80–85%). Amylose is a linear chain of several hundred glucose molecules, whereas Amylopectin is a branched molecule composed of thousands of glucose units (each chain of 24–30 glucose units is one unit of Amylopectin).
Water does not dissolve starches. By severing the alpha-linkages, they can be digested (glycosidic bonds). Humans and other animals both have amylases, which allow them to digest starches. In the human diet, potatoes, rice, wheat, and maize are major sources of starch. Starch formations are the means by which plants store glucose.
Glycogen is the supplementary long-term energy storage in animal and fungal cells, with adipose tissue holding the primary energy stores. Glycogen is primarily produced by the liver and muscles, but it can also be produced by glycogenesis in the brain and stomach. Glycogen is similar to starch, a glucose polymer found in plants, and is sometimes referred to as animal starch, with a structure similar to amylopectin but more extensively branched and compact than starch.
Glycogen is discovered in the cytosol/cytoplasm of many cell types as granules and plays an important role in the glucose cycle. Glycogen is an energy reserve that can be quickly mobilised to meet a sudden need for glucose, but it is less compact and more readily available as an energy reserve than triglycerides (lipids).
Galactogen would be a galactose polysaccharide that serves as an energy storage polysaccharide in pulmonate snails and some Caenogastropoda. This polysaccharide is found only in the albumen gland of the female snail reproductive system and in the perivitelline fluid of eggs, and it is unique to reproduction.
Galactogen acts as an energy reserve for developing embryos and hatchlings, and is eventually replaced by glycogen in juveniles and adults.
Structural polysaccharides:
Arabinoxylans seem to be copolymers of two sugars, arabinose and xylose, and are found in both primary and secondary cell walls of plants and they might be useful to human health.
In fact, plants’ structural components are primarily composed of cellulose. Wood is mostly cellulose and lignin, whereas paper and cotton are almost entirely cellulose. Cellulose is a polymer composed of repeated glucose units held together by beta-links. Because humans and many animals lack the enzyme required to break the beta-linkages, cellulose cannot be digested.
Termites, for example, can digest cellulose because bacteria containing the enzyme are present in their gut. Water does not dissolve cellulose. When mixed with iodine, it does not change colour. It produces glucose when hydrolyzed. It is the most common carbohydrate found in nature.
Chitin is just one of many polymers found in nature. Many animals, such as exoskeletons, have it as a structural component. It biodegrades in the natural environment over time. Its decomposition may be catalysed by chitinases, which are secreted by microorganisms such as bacteria and fungi and produced by some plants.
A few of these microorganisms have receptors for simple sugars derived from chitin decomposition. If chitin is detected, enzymes are produced to digest it by cleaving glycosidic bonds and converting it to simple sugars and ammonia.
Pectins are indeed a complex polysaccharide family with 1,4-linked -d-galactosyl uronic acid residues. They can be found in most primary cell walls as well as nonwoody parts of terrestrial plants.
Types of polysaccharides
There are two kinds of polysaccharides:
- Homopolysaccharides: A homopolysaccharide is a polysaccharide that includes the same type of monosaccharide. Glycogen, Cellulose, Starch, and Inulin are examples of important homopolysaccharides. Some other homopolysaccharides are pentosans (levans composed of arabinose or xylose) from woods, nuts, and other plant products, and fructans (levans) composed of fructose, such as inulin from Jerusalem artichoke and dahlia roots and tubers. Ivory nuts, orchid tubers, pine trees, fungi, and bacteria all contain mannose homopolysaccharides.
- Heteropolysaccharides: A heteropolysaccharide is a polysaccharide that includes different types of monosaccharides. Hyaluronic acid, Heparin, Chondroitin-4-sulfate, and Gamma globulin are examples of important heteropolysaccharides.
FAQs:
What type of compound is polysaccharide?
Polysaccharides, like the name implies, are large high-molecular-weight molecules formed by joining monosaccharide units together via glycosidic bonds. Glycans are another name for them. The three most important compounds in this class, cellulose, starch, and glycogen, are all glucose polymers.
What is heterogeneous polysaccharide?
Polysaccharides are frequently quite heterogeneous, with minor variations in the repeating unit. These macromolecules can have properties that differ from their monosaccharide building blocks depending on their structure.
What is storage polysaccharides and its types?
Each and every polysaccharide that functions as a source of stored energy in living organisms is known as storage polysaccharides. This include starch, phytoglycogen, and fructosans, while animal storage polysaccharides include glycogen.









