Table of Contents
Whenever a positively charged atom, known as a cation, attracts a negatively charged atom, known as an anion, salts are formed. An ionic bond is a positive to negative attraction that is essential for salts to maintain their chemical structure. Sodium chloride is one of the most significant salts in nature and biological systems. Sodium is an electrolyte that controls the amount of water in your body. Sodium has an impact on nerve impulses and muscle contractions. Sodium chloride is a salt substitute that is used to cure or prevent sodium loss due to dehydration, excessive sweating, or other reasons. Sodium chloride is said to be the chemical term for salt. Sodium is an electrolyte that controls the amount of water in your body. Sodium is a vital nutrient for human health as an electrolyte and osmotic solute. Excessive salt consumption in children and adults has been linked to an increased risk of cardiovascular disorders including hypertension. The health consequences of salt have long been researched.
Overview
In general, salts are considered the source of almost all chemical compounds that contain chlorine or sodium. It can be found in abundance in nature. Salt is a significant component of the dissolved components in seawater. Pure salt can be made from the mineral halite. Running water through the deposits gives brine solution, whereas mining the deposits yields sodium chloride. The salts dissolve as a result, and the solution is pushed out.
Presently, the evaporation of seawater is one of the most popular ways to obtain salt, and it is widely used in countries like India. Calcium sulphate, sodium sulphate, and other impurities are typically found in crystals. Pure crystals can be obtained by dissolving the salts in a little amount of water and filtering the solution.
Sodium Chloride
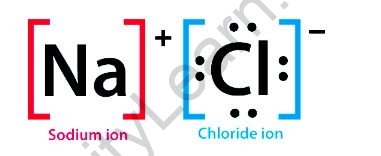
In sodium chloride, an ionic molecule, the sodium, and chloride ions are in a 1:1 ratio. It’s also known as table salt, common salt, or halite (the mineral form of common salt).
The salinity of saltwater, as well as the extracellular fluid present in many multicellular organisms, is mostly due to salt. It is utilized in a variety of processes ranging from domestic to industrial. This salt is primarily obtained from seawater. We can say that sodium chloride makes up about 1% to 5% of the water in the ocean. It’s a crystalline solid that’s white in colour. Aqueous saline solution is what it’s called.
NaCl is the chemical formula for sodium chloride.
This is a water-soluble molecule with a sodium cation and a chloride anion. It is commonly referred to as table salt and is mostly utilized in the food sector for preservation and flavouring. NaCl has a pH value of 7.
Preparation of Sodium Chloride:
Sodium and chlorine interact to generate sodium chloride, popularly known as table salt or common salt, which is widely used.
2Na(s)+Cl2( g)→2NaCl(s)
Physical Properties of Sodium Chloride:
The density of white crystalline solid sodium chloride is 2.165 g/mL, with a melting point of 801 °C and a boiling temperature of around 1,413 °C. Saline solutions, which are aqueous solutions with varying concentrations, are also available.
Chemical Properties of Sodium Chloride:
In water and other polar solvents, sodium chloride is an easily soluble chemical that is a stable solid. Only at very high temperatures does it break down, releasing deadly vapours of disodium oxide (Na2O) and hydrochloric acid (HCl).
Sodium Chloride in Water
When table salt is mixed with water, the Na and Cl atoms, which were formerly linked together in the form of a crystal, are dissolved by water molecules. A solvent is water.
The causes are purely electrostatic. Electrostatic bonds between charged or polar particles are what give atoms and molecules their cohesiveness. Sodium chloride (NaCl) is made up of two ions, Na+ and Cl–that are attracted to one another via electrostatic attraction.
Although water molecules are electrically neutral, their geometry leads them to be polarised, which means that the positive and negative charges are arranged in different directions. Under the stronger pulls produced by the water molecules, this characteristic causes the Na+ and Cl- ions to break apart. It’s worth noting that the orientation of the water molecules while attracting a Na+ ion differs from that when attracting a Cl– ion.
This procedure is repeated until the salt is completely dissolved.
Uses of Sodium Chloride
- It is known that table salt is sodium chloride, which is commonly used in the food sector for flavouring and preservation.
- It can be used to make things like sodium hydroxide, baking soda, sodium carbonate, hydrochloric acid, and other vital chemicals.
- It can be used in the textile, oil, paper, and pulp sectors, as well as fire retardants, road building, and rubber industries.
- The Solvay process is used in the soda ash business to manufacture sodium carbonate.
- In snowy and cold climates, sodium chloride is also used to de-ice roads and sidewalks. In medicine, these saline solutions are used for a variety of purposes.
- Cleaning items like shampoo and toothpaste, as well as water softeners, include the molecule.
Usage of Sodium Chloride for a Human Body:
- Nutrient Transportation and Absorption: In our small intestine, salt and chloride both play an important function. \ Sodium facilitates the body’s absorption of sugar, water, chloride, and amino acids (which are the building blocks of protein). When chloride is in the form of hydrochloric acid, it functions as a component of gastric juice (hydrogen and chloride). It aids in the digestion and absorption of nutrients in our bodies.
- For Maintaining Resting Energy: Sodium and potassium are electrolytes found in the fluid inside and outside of our body cells. The balance between these two particles determines how the cells retain the body’s energy. This also influences how nerves communicate with the brain, muscles contract, and heartbeats.
- For maintaining Hydration and Blood Pressure: In general, the brain, kidneys, and adrenal glands work together to maintain the body’s sodium levels. Chemical signals cause the kidney to retain water, allowing excess water to be excreted in the urine or reabsorbed into the circulatory system. While we have too much sodium in our blood, the brain tells the kidneys to release more water into circulation. As a consequence, blood volume and blood pressure rise. Once you reduce your salt intake, you absorb less water into your system. As a result, your blood pressure will fall.
Also read: Preparation and Properties of Sodium hydroxide
FAQs
What are the uses of the saline solution?
The most common term for a saline solution is regular saline, but it is also known as isotonic or physiological saline. In medicine, saline is used for a variety of purposes. It's used to treat dehydration, clean wounds, and clear sinuses. It can be applied topically or administered intravenously.
Does sodium chloride kill bacteria?
Sodium chloride is indeed not useful for a variety of purposes, but it is also an effective antibacterial agent. Antibacterial agents are said to be the substances that inhibit the growth and multiplication of bacteria.
Why is the formula of sodium chloride NaCl?
Generally, sodium chloride is generated when sodium atoms mix with chlorine atoms. Then, sodium will transfer an electron (a negatively charged particle) to chlorine and the chemical formula for sodium chloride is NaCl, which means that each sodium atom has exactly one chloride atom.









