Table of Contents
Introduction
In the century since the first industrial quantities of aluminum were produced, global demand for aluminum has increased to around 29 million tonnes per year. There are approximately 22 million tonnes of new aluminum and 7 million tonnes of recycled aluminum scrap. The use of recycled aluminum is both economically and environmentally advantageous. 1 tonne of new aluminum requires 14,000 kWh of energy to produce. In contrast, it only takes 5% of this to remelt and recycle one tonne of aluminum. There is no quality difference between virgin and recycled aluminum alloys. We can say that aluminum is a soft, ductile, corrosion-resistant metal with high electrical conductivity. It is widely used for foil and conductor cables, but alloying with other elements is required to provide the higher strengths required for these applications.
Overview
In general, aluminum is one of the lightest engineering metals, with a strength-to-weight ratio that exceeds that of steel. Aluminum is being used in an increasing number of applications by combining its advantageous properties such as strength, lightness, corrosion resistance, recyclability, and formability. This range of products includes everything from structural materials to thin packaging foils.
The primary ore bauxite is used to extract aluminum. There are significant bauxite deposits in Australia, the Caribbean, Africa, China, and South America. Bauxite is commonly mined using open-cut techniques. The Bayer process is used to purify the bauxite. This method involves dissolving aluminum trihydrate to produce alumina as well as iron and titanium oxides. Iron and titanium oxides are by-products of the process that are commonly referred to as ‘red mud.’ Red mud must be disposed of with extreme caution in order to protect the environment. One tonne of alumina requires approximately two tonnes of bauxite.
Principles of extraction of aluminum
The reactivity of aluminum is too high to extract it from its ore using carbon reduction. The temperatures required are far too high to be economically viable. Instead, electrolysis is used to extract it. The Bayer Process initially transfers the ore into pure aluminum oxide, which is then electrolyzed in solution in molten cryolite – another aluminum compound. Aluminum oxide has a melting point that is too high to electrolyze on its own. Bauxite is the most common aluminum ore. Bauxite is a type of impure aluminum oxide. Iron oxides, silicon dioxide, and titanium dioxide are the most common impurities.
Extraction of aluminum
Aluminum is by far the most abundant metal in the earth’s crust, accounting for 8% of the crust’s mass, and the third most abundant element after oxygen and silicon. Until now, the primary source of aluminum has been bauxite ore, which is a mixture of hydrated aluminum oxide.
Cryolite and alunite can also be used to recover aluminum. It can also be found in garnet, topaz, and chrysoberyl. This metal’s chemical symbol is Al. It is known that aluminum is a chemical element that belongs to the boron group and has the symbol Al and is the most commonly used non-ferrous metal.
The primary ore from which aluminum is extracted is bauxite. It is composed of a reddish rock clay-like material known as laterite soil and it is most common in tropical or subtropical climates. Aluminum oxide compounds (alumina), silica, iron oxides, and titanium dioxide make up bauxite. The electrolysis of alumina yields aluminum. Bayer’s process is used to concentrate bauxite ore into alumina. The Hall–Héroult electrolytic process is then used to refine alumina into pure aluminum metal.
Extraction of Aluminium: Bayer’s Process
Carl Josef Bayer’s Bayer Process is the primary industrial method of refining bauxite to produce alumina (aluminum oxide). Aluminum ore is treated with concentrated sodium hydroxide to form soluble sodium aluminate in this process. When sodium aluminate is filtered, the filtrate yields aluminum hydroxide when heated with water. When aluminum hydroxide is heated vigorously with water, it produces alumina.
Steps:
(a) Reaction with Sodium Hydroxide Solution:
- The crushed, as well as pulverized bauxite, is treated with a moderately concentrated sodium hydroxide solution (caustic soda), resulting in a slurry (a watery suspension) containing very fine ore particles.
- The slurry is pumped into a digester, which serves as a pressure cooker. The temperature in the digester is kept between 140°C and 240°C, and the pressure is kept between 35 atmospheres. These conditions are maintained for a period of time ranging from 30 minutes to several hours. To ensure that all aluminum-containing compounds are dissolved, more caustic soda may be added.
- When temperatures exceed 100 degrees Celsius, high pressures are required to keep the sodium hydroxide solution liquid. The higher the pressure required, the higher the temperature.
- The aluminum oxide combines or reacts with hot concentrated sodium hydroxide solution to form sodium tetrahydroxoaluminate solution.
- This procedure also dissolves silica, resulting in the formation of sodium silicate.
- Other metal oxides present as impurities in bauxite, on the other hand, do not react with the sodium hydroxide solution and thus remain unchanged. The slurry is pumped into a settling tank, where impurities that do not dissolve in the caustic soda settle to the vessel’s bottom.
- Every one of these solids is separated from the sodium tetrahydroxoaluminate solution via filtration, which is typically accomplished with a rotary sand trap. To remove the fine particles, a flocculant such as starch is added. They combine to form “red mud,” which is simply stored in vast lagoons.
(b) Precipitation of Aluminium Hydroxide:
The established crystals settle to the tank’s bottom and are removed. After that, the crystals are transferred to rotary kilns or fluid flash calciners. In these Kilns, aluminum hydroxide crystals are heated to 2,000 degrees Fahrenheit (1,100 degrees Celsius) to drive off the water molecules chemically bonded to the alumina molecules.
(c) Formation of Pure Aluminium Oxide:
The established crystals settle to the tank’s bottom and are removed. After that, the crystals are transferred to rotary kilns or fluid flash calciners. Aluminum hydroxide crystals are heated at a temperature of 2000F (1100C) in these Kilns to drive off the water molecules chemically bonded to the alumina molecules.
For bauxites containing more than 10% silica as an impurity, the Bayer process becomes uneconomic due to the formation of insoluble sodium aluminum silicate, which reduces yield, so a different process must be used.
The extraction is accomplished through electrolysis.
Extraction of Aluminium: Hall-Heroult Process
In general, the Hall–Héroult process is a large industrial process involving the smelting of aluminum. It entails dissolving aluminum oxide (alumina) derived from bauxite via the Baeyer process. Pure alumina is mixed with calcium fluoride (CaF2) or cryolite in this process (Na3AlF6). Elemental aluminum cannot be produced by the electrolysis of an aqueous aluminum salt because hydronium ions oxidize elemental aluminum.
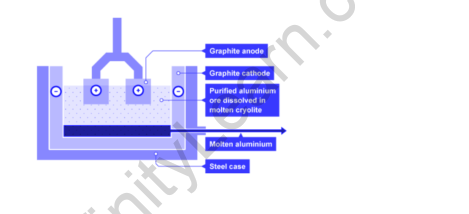
A steel vessel with a carbon lining is used in the arrangement. The cathode (negative electrode) is the carbon lining, and the anode is graphite rods (positive electrode). Rods suspend the anodes in the molten alumina-cryolite mixture.
When electricity is passed through the cell, oxygen is formed at the anode. The formed oxygen reacts with the carbon anode to produce carbon monoxide and carbon dioxide. The cell operates at a low voltage of approximately 5–6 volts but at massive currents of 100,000 amps or more. The heating effect of these large currents keeps the cell at around 1000 C.
Because aluminum is heavier than cryolite, it settles at the bottom of the tank and is periodically siphoned out. To maintain a sufficient amount of dissolved alumina in the bath, alumina is fed into the pots at regular intervals to sustain the electrolytic process. Approximately 430kg of carbon is consumed for every tonne of aluminum produced. Both smelters produce anodes in dedicated carbon plants on a continuous basis.
Electrolysis of aluminum
Electrolysis is the process of using electricity to break down electrolytes in order to form elements and the electrolysis products can be predicted for a given electrolyte. Aluminium is one of the metals extracted from its ore using this method.
Bauxite is the name given to aluminum ore (Al2O3). The bauxite is purified to produce a white powder containing aluminum oxide (also known as alumina), from which aluminum can be extracted.
The extraction is accomplished through electrolysis, but the aluminum oxide must first be melted in order for electricity to pass through it. However, because aluminum oxide has a very high melting point (over 2,000°C), melting it would be costly.
It’s then dissolved in molten cryolite, an aluminum compound with a lower melting point than aluminum oxide. By allowing the ions in aluminum oxide to move freely at a lower temperature, the use of molten cryolite as a solvent reduces some of the energy costs associated with extracting aluminum.
An aluminum oxide electrolysis cell is depicted in the diagram. Both the negative (cathode) and positive (anode) electrodes are made of graphite, a type of carbon.
Aluminum ions are reduced to aluminum atoms after receiving electrons at the negative electrode:
Al3+ + 3e– → Al (reduction – gain electrons)
The molten form drains to the cell’s bottom and is tapped off.
At the positive electrodes, oxide ions lose electrons and are oxidized to oxygen gas:
2O2– → O2 + 4e– (oxidation – lose electrons)
The whole oxygen combines with the carbon of the positive electrodes to form carbon dioxide, causing them to slowly burn away. So, the positive electrodes must be replaced on a regular basis. This raises the overall cost of the procedure.
The information about the principles of extraction of aluminum from various chemistry-related articles is available here. The primary ore bauxite is used to extract aluminum. Students who want to flourish in chemistry need to be well known about this to get deep knowledge about it to do well on their exams. The principle and steps in aluminum extraction are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional chemistry help.
FAQ’s
What is the process of aluminum extraction?
Bauxite is the name given to aluminum ore and it is purified to yield aluminum oxide, a white powder from which aluminum can be extracted. Electrolysis is used for extraction.
Why is cryolite used in the extraction of aluminum?
By allowing the ions in aluminum oxide to move freely at a lower temperature, the use of molten cryolite as a solvent reduces some of the energy costs associated with extracting aluminum.
How do you extract aluminum from the soil?
The extraction of exchangeable forms of soil aluminum is commonly done with a salt solution of strong acid, whereas the determination of soluble aluminum in soils is done with a salt solution of a weak acid.








