Table of Contents
Sulfuric acid (H2SO4), often known as oil of vitriol or hydrogen sulfate, is a viscous, colorless, oily, caustic liquid that is one of the most economically important compounds. Sulfuric acid is created industrially by reacting water with Sulphur trioxide, which is made by combining Sulphur dioxide and oxygen in a chemical reaction using either the contact or chamber technique. Fertilizers, pigments, dyes, medicines, explosives, detergents, inorganic salts and acids, as well as petroleum refining and metallurgical operations, all utilize the acid in varying quantities. Sulfuric acid is used as the electrolyte in lead-acid storage batteries, which is one of its most well-known applications.
A brief outline
Sulfuric acid is a highly powerful acid that totally ionizes in aqueous solutions to create hydrogen ions (H3O+) and hydrogen sulfate ions (HSO4). Hydrogen sulfate ions break down in dilute solutions, creating additional hydronium ions and sulfate ions (SO4[-2]). Concentrated sulfuric acid is a strong dehydrating agent, combining violently with water, and chars many organic materials, such as wood, paper, or sugar, leaving a carbonaceous residue, in addition to being an oxidizing agent, reacting readily at high temperatures with many metals, carbon, Sulphur, and other substances.
Mostly in the chemical sector, sulfuric acid is also significant. It’s most frequent in fertilizer production, but it’s also employed in mineral processing, oil refining, wastewater treatment, and chemical synthesis. It’s used in a variety of products, including acidic drain cleaners for the home, as an electrolyte in lead-acid batteries, to dehydrate a chemical, and as a cleaning agent. Sulfur trioxide can be made into sulfuric acid by dissolving it in water.
Important concepts
Use of sulphuric acid
-
Chemical manufacturing in the industrial sector
Sulfuric acid is mostly utilized in the “wet technique” of producing phosphoric acid, which is used to make phosphate fertilizers. Phosphate rock is used in this technology, and about 100 million tons are processed each year. Although the actual composition of this raw material is unknown, it is indicated below as fluorapatite. Calcium sulfate, hydrogen fluoride (HF), and phosphoric acid are produced by treating this with 93 percent sulfuric acid. Hydrofluoric acid is used to eliminate HF.
-
The cycle of sulfur and iodine
The sulfur–iodine cycle is a set of thermochemical reactions that could be used to generate hydrogen from water. It comprised of 3 chemical reactions with water as the net reactant and hydrogen and oxygen as the net products. Because the sulfur and iodine compounds are collected and reused, the process is referred to as a cycle. Because this is an endothermic process that must take place at high temperatures, energy in the form of heat must be provided. The sulfur–iodine cycle is being investigated as a viable means of generating hydrogen, but the concentrated, corrosive acid at extreme temps now offers insurmountable safety risks if the process were to be developed on a big scale.
-
Cleaning agent for industrial use
The iron and steel industry uses sulfuric acid in enormous quantities to remove oxidation, rust, and scaling in rolled sheets and billets before selling them to the automobile and big appliance industries. A spent acid regeneration (SAR) facility is frequently used to recycle used acid. These facilities use natural gas, refinery gas, fuel oil, or other fuel sources to burn waste acid. Sulfur dioxide (SO2) and sulfur trioxide (SO3) are produced during the combustion process, which is subsequently used to make “new” sulfuric acid.
Concentrated sulphuric acid
When pure 100 percent acid is heated, sulfur trioxide gas, SO3, is released, resulting in a constant-boiling solution, or azeotrope, comprising around 98.5 percent H2SO4 at 337°C. Because so little of it dissociates into ions at ambient temperature, concentrated sulfuric acid is a weak acid and a poor electrolyte. It does not interact quickly with ordinary metals like iron or copper when it is cold. When heated, it acts as an oxidizer, reducing the sulfur content; sulfur dioxide gas may be produced. Most metals and numerous nonmetals, such as sulfur and carbon, react with hot concentrated sulfuric acid. When sodium chloride (NaCl), or common salt, is cooked with strong sulfuric acid, hydrogen chloride gas, HCl, is produced.
Sulfuric acid in concentrated form has a high attraction to water. It may be used to dehydrate numerous substances, including carbohydrates, and is sometimes employed as a drying agent. It combines with sucrose, taking eleven molecules of water (H2O) from each molecule of sucrose and leaving a brittle, spongy black mass of carbon and diluted sulfuric acid in its place. Skin, cellulose, and other plant and animal materials react similarly to the acid.
Colour of Sulphuric acid
At normal temperature, sulfuric acid is an oily liquid. It is colorless, but when slightly polluted with iron ions, it takes on a very pale-yellow hue. Concentrated sulfuric acid can turn pale yellow or pink, red, brown, or even black when minute amounts of dissolved organic matter are present.
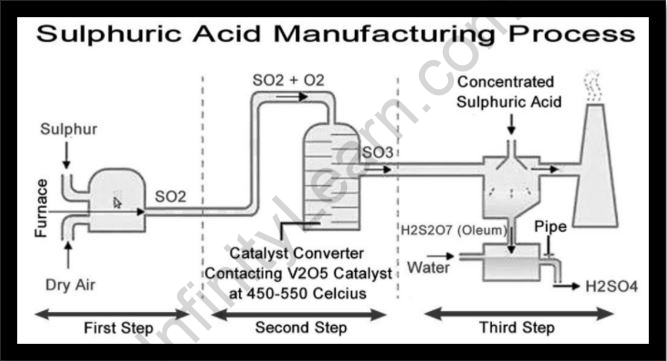
The industrial process for producing sulphuric acid
The following are the steps involved in the production of sulphuric acid:
- Sulfur dioxide preparation.
- Sulfur dioxide is converted to sulfur trioxide.
- Sulfur trioxide is generated, and it is converted into concentrated H2SO4.
Step 1: Sulphur dioxide production: SO2 is produced by burning sulfur in the presence of extra air, resulting in a product that mixes with oxygen, which is useful for the next stage.
S(s) + O2 (g) → SO2(g)
Step 2: Sulfur trioxide is generated when sulfur dioxide interacts 1:1 with oxygen at a temperature of 400°C–450°C and a pressure of 1-2 atm in the vicinity of V2O5 as a catalyst at a temperature of 400°C–450°C and a pressure of 1-2 atm. In nature, this reaction is reversible.
2SO2(g) + O2(g) ⇌ 2SO3(g)
Step 3: Concentrated sulphuric acid preparation
The resulting sulfur trioxide is then used to react with concentrated sulphuric acid. Sulfur trioxide cannot be directly dissolved in water because it causes fog to arise. The end product of this reaction is known as oleum. After that, the oleum is dissolved in water to make concentrated sulphuric acid.
H2SO4 + SO3(g) → H2S2O7(l)
H2S2O7(l) + H2O(l) → 2H2SO4
Pure anhydrous sulfuric acid doesn’t really exist in nature due to its affinity for water. Depending on the emissions associated with individual volcanoes, volcanic activity can result in the formation of sulfuric acid, and sulfuric acid aerosols from an eruption can stay in the stratosphere for many years. Although volcanic activity is a modest contribution to acid rain, these particles can convert into sulfur dioxide (SO2), an ingredient of acid rain.
Significance of Sulphuric acid manufacture in NEET exam
The expert or teacher of infinity learn places ahead of their greatest exertion without fail to make their notes, and they ensure that all points of view are tended to totally so students get the most significant expected engraves on their NEET test. Model request reactions to are depicted comprehensively with immaculate clarifications for each part to ensure that students get the idea. Each number-related issue is settled one by one, with no movement bobs, which is straightforward for young people to recognize. Students taking NEET tests will benefit exceptionally from the parts recorded beforehand.
FAQ’s
Between H2O and H3O+ and -OH, water forms a compromise. Nothing is ionic as a result of this. Because all of the bonds in sulfuric acid are covalent, it is a covalent chemical. The fact that it ionizes easily is beside the point.
Pour baking soda immediately into an acid spill. Light acids like vinegar, as well as dangerous, powerful acids like muriatic and sulphuric acids, will be neutralized by this method. To neutralize the acid, cover the entire contaminated area with baking soda (sodium bicarbonate).
The most common use of sulfuric acid is in fertilizer production. It's used to make sulfate salts, synthetic detergents, and pigments, among other things. Is sulfuric acid molecular or ionic?
What is the best way to neutralize sulfuric acid?
What is the purpose of sulfuric acid?








