Table of Contents
Chlorine, with the atomic number 17 and the symbol Cl, is a chemical element. It is the second-lightest of the halogens, appearing between fluorine and bromine in the periodic table, and its characteristics are generally halfway between them. Chlorine is a yellow-green gas at normal temperature. It is a highly reactive element and a powerful oxidizer, with the greatest electron affinity and third-highest electronegativity on the updated Pauling scale, behind only oxygen and fluorine. Nitrogen’s electronegativity is listed as greater than chlorine’s on several scales other than the revised Pauling scale, including the Allen, Allred-Rochow, Martynov-Batsanov, Mulliken-Jaffe, Nagle, and Noorizadeh-Shakerzadeh electronegativity scales. Due to its extreme reactivity, all chlorine in the Earth’s crust is in the form of ionic chloride compounds, which include table salt. It is the second most abundant halogen in the Earth’s crust (after fluorine) and the twenty-first most abundant chemical element. Despite this, the vast reserves of chloride in seawater dwarf these crustal deposits. Chlorine, in the form of chloride ions, is required by all known species of life. Other types of chlorine compounds are uncommon in living organisms, and chlorinated organics produced artificially range from inert to toxic. Chlorine-containing organic molecules, such as chlorofluorocarbons, have been linked to ozone depletion in the upper atmosphere. As part of the immune system’s response to bacteria, neutrophils produce small amounts of elemental chlorine through the oxidation of chloride ions.
Hydrochloric acid, commonly known as muriatic acid, is a hydrogen chloride aqueous solution. It’s a clear liquid with a strong, unpleasant odour. It’s categorised as a strong acid. It is a component of gastric acid in most animal digestive systems, including humans. Hydrochloric acid is a widely used laboratory reagent and chemical in industry. The salt of protonated water and chloride is hydrochloric acid. Its ions are frequently written as H3O+ Cl, despite the fact that the cation is frequently bonded to other water molecules. A combined IR, Raman, X-ray, and neutron diffraction study of concentrated hydrochloric acid revealed that the primary form of H+(aq) in these solutions is H5O2+, which is hydrogen-bonded to neighbouring water molecules in several ways, along with the chloride anion.
Overview of Chlorine and Hydrochloric Acid
In seawater, salt lakes, and brine deposits, chlorine exists as chlorides. Chlorine gas is manufactured in the industrial sector by electrolysis of sodium chloride solutions. It is also produced as a byproduct of the electrolysis of molten salts such as NaCl, MgCl2, and CaCl2. Chlorine, as an element, is an oxidizer. It reacts with lower halide salts to undergo halogen substitution reactions. For example, chlorine gas can be bubbled through a bromide or iodide anions solution. These anions are oxidised by the gas to bromine and iodine, respectively. Chlorine also participates in free-radical substitution reactions with organic compounds containing hydrogen. Because chlorine is toxic to bacteria, some of which can cause disease, it is used to purify water supplies. As a result, incorporating it into water supplies benefits the population. Because chlorine is toxic to bacteria, some of which can cause disease, it is used to purify water supplies. As a result, incorporating it into water supplies benefits the population. Drinking water chlorination raises concerns about individual freedom because it makes it difficult for people to opt-out. Chlorine is also used in disinfectants, PVC, and chloroform construction, and as an oxidising agent in many pharmaceutical products.
Hydrochloric acid is a colourless, odourless, and highly corrosive hydrogen chloride and water solution. HCl can only dissociate once and lose one proton. HCl is one of the most powerful commercially available cleaners today. It is extremely powerful and is used in masonry, glue production, and as a bleaching agent in the textile industry. It is also used to purify ordinary salt. HCl can be made in the laboratory by heating sodium chloride (NaCl) with concentrated H2SO4. The produced gas (HCl) is dried by passing it through conc. H2SO4 and then mixed with water to produce the acid.
Hydrochloride Acid
When Hydrogen Chloride gas is mixed with water, it produces hydrochloric acid. When the reaction occurs, hydrochloric acid, also known as muriatic acid, forms, emitting a pungent odour and resulting in an odourless and colourless solution. Each molecule of HCl is made up of hydrogen and chlorine in a one-to-one ratio. It is a highly corrosive chemical compound that can also be toxic. Hydrochloric acid, also known as muriatic acid, is a poisonous, corrosive hazardous liquid that reacts with most metals to produce explosive hydrogen gas, causing severe burns and irritation of the eyes and mucous membranes. It’s created by soaking hydrogen chloride in water. The majority of acid is a byproduct of chlorination. The combustion of chlorine and hydrogen produces pure acid. Technical, recovered, food processing, and reagent grades of hydrogen acid are all available. Because of impurities such as dissolved iron, commercial grades are frequently slightly yellow. Reagent grade, which contains approximately 37.1 percent hydrochloric acid, is completely clear and colourless.
Hydrochloric acid is a powerful, corrosive acid that can be used to treat steel in the building and construction sectors. It is used in the chemical industry to make vinyl chloride, which is used to make polyvinyl chloride (PVC) plastic, and it is one of the chemicals used to make polyurethane foam and calcium chloride. Hydrochloric acid is also used to make many other chemicals, as well as a disinfectant and slimicide, which is a chemical that prevents slime growth in paper stock.
Use of Chlorine
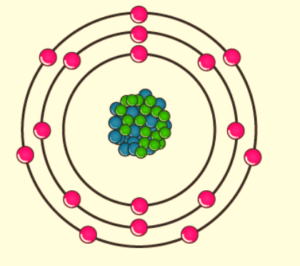
Chlorine, a nonmetal in group 17, is the second halogen in the periodic table. As a result, its properties are similar to those of fluorine, bromine, and iodine, and are generally intermediate between the first two. Chlorine has the electron configuration [Ne]3s23p5, with the seven electrons in the third and outermost shell serving as its valence electrons. As with all halogens, it is one electron short of a full octet, making it a powerful oxidising agent that interacts with other elements to complete its outer shell.
- It is used to eliminate the odour of putrefaction.
- It is employed as a disinfectant.
- To kill bacteria, chlorine is used in the treatment of drinking water.
- It is employed in the cleaning of swimming pools.
- It is used in the manufacture of paper and paper-related products.
- It is employed as an antiseptic.
- It is used in the manufacture of drugs.
- It’s used in the production of dyes and plastics.
Chlorine and Sodium Hydroxide
The following is the reaction between chlorine and cold dilute sodium hydroxide solution:
![]()
NaClO (also spelled NaOCl) is sodium chlorate (I). The old name for this is sodium hypochlorite, and the solution on the right-hand side of the equation is commonly known as bleach.
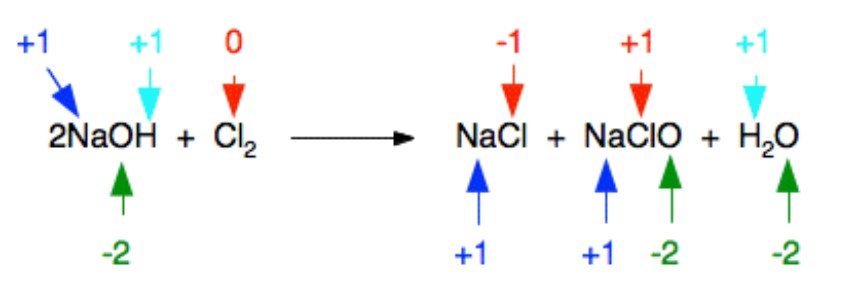
Because its oxidation state has changed, one atom has been reduced. The other has oxidised. This is an excellent illustration of a disproportionation reaction. A disproportionation reaction occurs when a single substance is oxidised and reduced at the same time.
FAQs Uses of Chlorine and Hydrochloric Acid
What is the chlorine period?
Chlorine is a periodic table element in group 17, also known as halogens, and is only found in nature as a compound, not as an element. The most common are salt (sodium chloride) and potassium compounds (sylvite).
Is chlorine flammable?
Although chlorine is unstable as a result of relatively unlikely secondary exposure from exposed individuals, chlorine gas can condense on the skin and contaminate others via dermal contact.
What is the purpose of hydrochloric acid?
Hydrochloric acid is also used in the production of batteries, photoflash bulbs, and fireworks. It is even used in the production of sugar and gelatin. Hydrochloric acid, like last months chlorine compound, sodium chloride, is a chemical workhorse because it is extremely useful in a wide range of applications.
Is hydrochloric acid harmful?
Hydrochloric acid is a toxic substance that is strictly controlled. Condensed hydrochloric acid, also known as hydrochloric acid fumes, may be present in acidic mist. Both the mist and the solution are corrosive to human tissue, causing irreversible damage to breathing organs, hair, skin, and intestines.




