Table of Contents
There is a distinction between alcohols based on whether or not they have hydroxyl groups. Alcohols have different physical and chemical properties depending on where the hydroxyl group is located. There are three sorts of alcohol. Primary, secondary, and tertiary alcohols are the three forms of alcohol. The position of an alkyl group’s carbon atom in relation to the hydroxyl group determines its classification. At room temperature, a number of alcohols are described as colorless liquids or even solids. The molecular weight of alcohol determines how soluble it is in water; the greater the molecular weight, the less soluble the alcohol is in water and the higher the density, boiling point, vapour pressure, and viscosity.
A Brief Outline
Primary alcohol
Alcohols with only one alkyl group connected to the hydroxyl group are referred to as primary alcohols (OH). Methanol (propanol), ethanol, as well as other main alcohols are examples. The intricacy of an alkyl chain has no bearing on whether it is classified as primary or secondary. For any alcohol to qualify as a primary, there must be only one bond between a –OH group and an alkyl group.
Secondary alcohol
Secondary alcohols usually have two adjacent alkyl groups and a carbon atom linked to the hydroxyl group. There could be two structurally identical or even two distinct alkyl groups.
Tertiary structure
Tertiary alcohol is defined as alcohol with a hydroxyl group bonded to the carbon atom and three alkyl groups connected to it. Because of their structure, these alcohols have different physical properties. The -OH group permits the alcohols to form hydrogen bonds with their surrounding atoms. Because of the weak connections established between the molecules, the boiling points of alcohols are higher than those of alkanes.
The oxidation test can also be used to perform alcohol identification tests. Alcohol is oxidized using sodium dichromate (Na2Cr2O7) in the oxidation test. Primary alcohol oxidation differs from secondary and tertiary alcohol oxidation. The oxidation rate distinguishes primary, secondary, and tertiary alcohols. Alcohols are classified as follows based on their oxidation rates. The primary alcohol is quickly converted to an aldehyde, which could then be converted to carboxylic acids. Secondary alcohol can easily be oxidized to a ketone, but no further oxidation is possible. In the presence of sodium dichromate, tertiary alcohol does not oxidize.
Important concepts
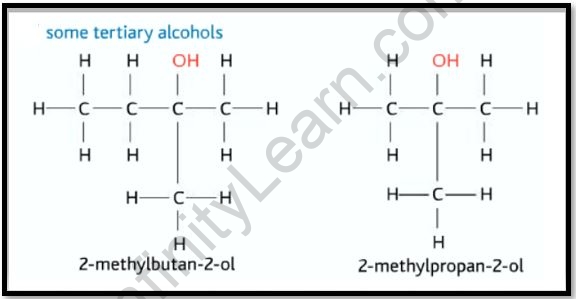
Tertiary alcohol examples and structure
2 Methyl butanol
The chemical molecule CH3CH2CH(CH3)CH2OH (IUPAC designation, often known as active amyl alcohol) has the formula CH3CH2CH(CH3) CH2OH. It’s one of several amyl alcohol isomers. It’s a colorless liquid that exists in trace amounts in nature and has sparked interest as a potential biofuel due to its hydrophobic (gasoline-like) and branching structure. It’s chiral in nature. Many commercial amyl alcohol mixes contain 2-methyl-1-butanol as a component. It’s one among numerous fragrance components found in many fungi and fruits, such as the summer truffle, tomato, and cantaloupe.
Genetically engineered E. coli has created 2-methyl-1-butanol from glucose. The enzymes 2-keto acid decarboxylase and dehydrogenase work together to convert 2-keto-3-methyl valerate, a precursor to threonine, to the target alcohol. It can be made from fuel oil (which is found naturally in fruits like grapes) or synthesized via the oxo method or pentane halogenation.
2 Methyl propanol (Isobutanol)
The chemical compound Isobutanol (IUPAC nomenclature: 2-methyl propane-1-ol) has the formula (CH3)2CHCH2OH (sometimes represented as i-BuOH). This colorless, flammable liquid with such a distinct odor is mostly utilized as a solvent, either directly or in the form of its esters. The other butanol, or isomers, are n-butanol, 2-butanol, and tert-butanol, which are all important industrially. The carbonylation of propylene produces Isobutanol. Hydroformylation, which produces a combination of isobutyraldehyde and butyraldehyde, is the more prevalent process used in industry.
Isobutanol and n-butanol have comparable applications. They’re frequently interchanged. Varnishes and precursors of esters, which are helpful solvents, such as isobutyl acetate, are the most common uses. Plasticizers such as isobutyl esters of phthalic, adipic, and similar dicarboxylic acids are widely used. Some biofuels contain Isobutanol as well.
Identifying Tertiary Alcohol
A few drops of alcohol are added to a potassium dichromate (VI) solution acidified with dilute sulfuric acid in a test tube. After that, the tube is warmed in a hot water bath. The colors are observed after heating. The orange solution turns green when it comes into contact with primary or secondary alcohols. To discriminate between the primary and secondary alcohols, Schiff’s test will be necessary. With tertiary alcohol, there is no colour change.
Significance of Identification of tertiary alcohol in NEET exam
Prepared experts and experienced educators in the subject give replies. The responses are as indicated by CBSE and NCERT models for the NEET test, helping understudies in accomplishing higher outcomes. The courses are sensibly regarded, and there are two or three free courses accessible for new understudies to test. On a relative stage, there is a substitute selection of courses going from kindergarten to twelfth grade, as well as express exercises for certifiable tests like NEET.
FAQs
Alcohols are naturally acidic. Alcohol has a stronger electronegative oxygen group linked to the carbon and hydrogen group than hydrogen. As a result, it pulls the electron cloud towards itself, causing deprotonation.
Only primary, secondary, and tertiary alcohols pass the Victor Meyer test. Phenols do not provide it. This is because, in the first step, R-OH is converted to R-I, but the procedure requires breaking the OH bond with carbon; however, the carbon-oxygen bond in phenol is much stronger, and even the creation of the transition state will totally rupture the aromaticity of the compound, rendering it unstable.
Test reagents that act with the -OH group are used to determine the presence of alcohol. To identify alcohol, take a clear, neutral liquid that is free of water and add a solid phosphorus (V) chloride. The presence of alcohol is indicated by a rush of acidic steamy hydrochloric vapours. To discriminate between alcohol classes, additional tests are required. Is it true that alcohols are acidic in nature?
What does the Victor Meyer Test tell you about?
How Can Alcohol Reactivity Help You Differentiate Between Classifications?









