Class 9 Osmosis and Diffusion in Cell
Table of Contents
- Osmosis – Types of Solutions
- Summary
- What’s Next?
In the previous segment of the chapter ‘Cell Structure and Functions’, we got introduced to the process of Osmosis. In this segment, let us learn more about the process of Osmosis and understand the transportation of molecules in a cell.
What are the Three types of osmotic solutions?
Osmosis is the process in which water moves from higher water concentration to lower water concentration through a semi-permeable membrane.
For example, the swelling of raisins when kept in water for some time.
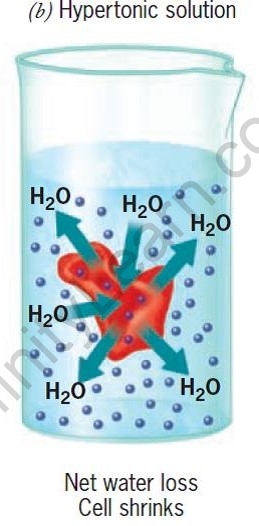 The movement of water is affected by the amount of the substance dissolved in it. Depending on the concentration of the solution, there are three types of osmotic solutions.
The movement of water is affected by the amount of the substance dissolved in it. Depending on the concentration of the solution, there are three types of osmotic solutions.
| Hypertonic Solution | Isotonic solution | Hypotonic Solution | |
| Water in the cell | More than in surrounding | Same as in surrounding | Less than in surrounding |
| Water in the surrounding | Less than in cell | Same as in cell | More than in cell |
| Cells | Shrink | No change | Swell |
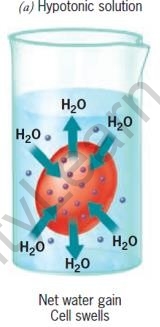
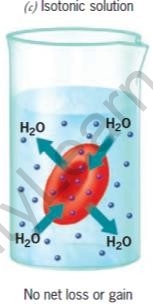
RBCs in different types of salt solutions

