Table of Contents
Table of Contents
- Rules for Writing Chemical Formula of Compounds
- Valency of Elements
- Examples of Chemical Formulae (Sharing of Electrons):
- Hydrogen Chloride
- Hydrogen Sulphide
- Summary
- Did You Know?
- What’s Next?
In the previous segment, we learned about the third rule for writing the chemical formulae of compounds based on valency.
In this segment, we will compile the three rules and learn how to write chemical formulae using them.
What are the rules for writing chemical formulae of compounds?
Rule 1 – In a compound formed by metal and non-metal, the metal component is written first and the non-metal later.
Rule 2 – Polyatomic ions in the compounds are represented using brackets.
Rule 3 – The net charge on a complete molecule should be balanced. In order to balance the net charge on a molecule, we require the valency of each element present in that molecule.
What is Valency of an element?
We know that Valency is the combining power of an element, that is related to the number of electrons present in its outer shell or valence shell. The valencies for some elements are shown below:
Valency of elements chart
|
Name of Element |
Symbol |
Valency |
|
Hydrogen |
H |
1 |
|
Helium |
He |
0 |
|
Lithium |
Li |
1 |
|
Beryllium |
Be |
2 |
|
Boron |
B |
3 |
|
Carbon |
C |
4 |
|
Nitrogen |
N |
3 |
|
Oxygen |
O |
2 |
|
Fluorine |
F |
1 |
|
Neon |
Ne |
0 |
|
Sodium |
Na |
1 |
|
Magnesium |
Mg |
2 |
|
Aluminium |
Al |
3 |
|
Silicon |
Si |
4 |
|
Phosphorus |
P |
3 |
|
Sulphur |
S |
2 |
|
Chlorine |
Cl |
1 |
We will now use the above rules to write the chemical formulae of hydrogen chloride and hydrogen sulphide.
Writing the chemical formula for compounds (Sharing of electrons)
Hydrogen chloride
The symbol for hydrogen is ‘H’ and chlorine is ‘Cl’. Both have a valency of one. Also, both
the hydrogen and chlorine have a tendency to acquire one electron.
Thus, one hydrogen atom combines with one chlorine atom to form hydrogen chloride by sharing one electron each.
Balancing the net charge, we get the chemical formula as ‘?1??1’.
But we need not write one in the chemical formula, hence the chemical formula for
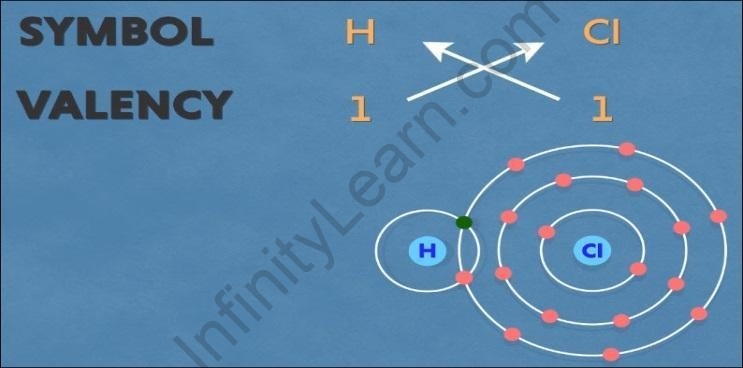 hydrogen chloride as ‘HCl’
hydrogen chloride as ‘HCl’




