Table of Contents
Table of Contents
- Compounds – Definition
- Ions – Definition
- Chemical Formulae
- Summary
- Did You Know?
- What’s Next?
In the last segment, we learnt about the concept of Molecules. In this segment, we will learn about molecules of compounds and the concept of Ions.
What are Compounds?
A Compound is simply two or more elements combined in a fixed proportion. In simple words, every compound is made up of molecules; and molecules are made up of different atoms in a fixed proportion.
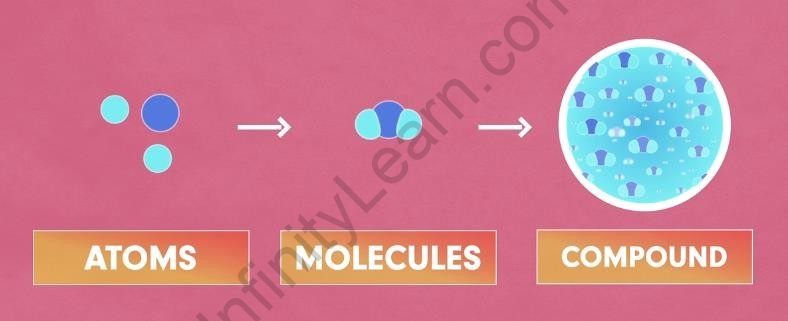
Compounds
For example, 2 hydrogen atoms and 1 oxygen atom come together to give ?2?, that is 1 molecule of the compound water. This means that in a water molecule, the ratio of hydrogen and oxygen will always be 2:1.
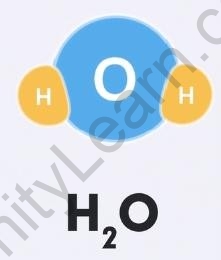
Water molecule
Here are a few more examples of atoms of different elements combining in a fixed proportion to give a molecule of a specific compound.
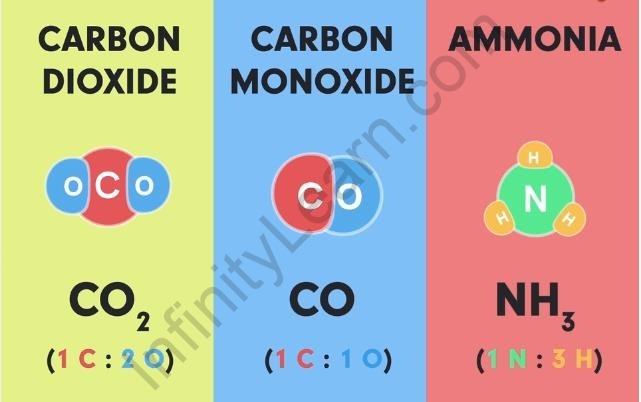
Examples of molecules of compounds
What are Ions?
An atom or group of atoms that carries a positive or negative electric charge as a result of having lost or gained one or more electrons are called Ions.
A positively charged ion is called a Cation and a negatively charged ion is called an Anion.
When a cation and an anion come closer, they attract each other and get bonded. This bonding leads to the formation of a molecule. Usually, with the formation of such a molecule, the net charge on it is neutral.
Many such similar molecules come together to form a compound.




