Table of Contents
Introduction to Formic Acid
Methanoic acid is also known as formic acid, which is a simple and important organic compound. It is widely used in various industries and has numerous applications due to its unique properties.
In this article, we will explore the structure, formula,uses, and properties of methanoic acid to gain a comprehensive understanding of this versatile substance. Let’s delve into the world of methanoic acid and discover its significance in different fields.
Formic Acid Structure and Formula
Methanoic acid formula class 10 is HCOOH which can be written as CH2O2. It consists of a carboxyl group (-COOH) attached to a single hydrogen atom. The carboxyl group is responsible for the acidic properties of the compound. The methanoic acid structure is shown below:
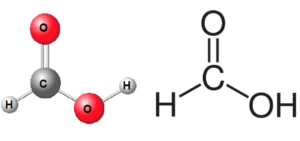
Occurrence and Sources
Naturally, Methanoic acid found in various organisms, including ants, bees, and certain plants. Ants produce this acid as a defense mechanism, while bees use it in their venom.
Additionally, methanoic acid found in the stings of some nettle plants. It is created by the reaction of sulfuric acid with sodium formate, which is created from carbon monoxide and sodium hydroxide.
Properties of Formic Acid
- Physical State: Formic Acid (Methanoic acid) is a colorless liquid at room temperature.
- Odor: It has a pungent odor, similar to vinegar.
- Solubility: It is highly soluble in water and most organic solvents.
- Corrosive Nature: Formic Acid is corrosive to metals and can cause burns to the skin.
- Reactivity: It is a strong reducing agent and can react with various substances.
Chemical Properties of Formic Acid
- Formic Acid converts mercuric chloride to mercurous chloride, resulting in a white precipitate. The chemical formula is shown below.
HCOOH + 2HgCl2 → Hg2Cl2 + 2HCl + CO2 - Phosphoric pentachloride interacts with Formic Acid to generate formyl chloride, phosphoryl chloride, and hydrogen chloride.
HCOOH + PCl5 → HCOCl + POCl3 + HCl
Formic acid uses
Formic acid has diverse applications in different industries:
- Textile Industry: It is used in dyeing and finishing processes for textiles.
- Leather Industry: It is utilized in the tanning process to preserve leather.
- Agriculture: Methanoic acid is used as a preservative in animal feed and silage.
- Cleaning Agent: It is employed in cleaning products due to its acidic properties.
- Food Industry: In the food sector, it serves as an acidulant and preservative.
Formic Acid (Methanoic acid) vs. Ethanoic Acid
Formic acid (methanoic acid) and ethanoic acid (acetic acid) are both carboxylic acids, but they have distinct properties and uses. The ethanoic acid formula is CH3COOH. Formic acid is a stronger reducing agent and is often used as a preservative in animal feed and silage, while ethanoic acid is commonly used in the food industry as a flavoring agent and preservative.
Conclusion
Methanoic acid, or formic acid, is a significant compound with various applications in different industries. Its unique structure and properties make it an essential chemical in nature and human activities.
Formic acid examples include its use as a preservative in food and animal feed, a tanning agent in the leather industry, a textile printing and dyeing agent, a cleaning agent, agricultural pesticides, and in the rubber industry for improved production processes and product quality.
Understanding its structure, formula, uses, and properties helps us appreciate its significance and versatility.
Frequently Asked Questions on Formic Acid
What is the structure of methanoic acid?
Methanoic acid has a simple structure consisting of a carboxyl group (-COOH) attached to a single hydrogen atom. This carboxylic acid group gives it its acidic properties.
What is the formula of methanoic acid?
The chemical formula of methanoic acid is HCOOH, and its molecular formula is CH2O2.
What are the main uses of methanoic acid?
Methanoic acid has diverse applications, including its use in the textile industry for dyeing and finishing, in the leather industry for tanning, in agriculture as a preservative for animal feed and silage, and as an acidulant and preservative in the food industry.
Is methanoic acid the same as formic acid?
Yes, methanoic acid is also known as formic acid. Both terms refer to the same compound with the chemical formula HCOOH.
Why does methanoic acid have the formula HCOOH?
Formic acid belongs to the carboxylic acid family. Formic acid is the first member of the carboxylic acid group and is written as HCOOH. Methanoic acid is another name for formic acid. To be more specific, the formic acid formula has two oxygen atoms, one carbon atom, and two hydrogen atoms.





