Table of Contents
Introduction:
Specific heat values can be calculated as follows: When two materials, each at a different temperature, come into contact with one another, heat always flows from the warmer material into the colder substance until they’re both at the same temperature. The heat gained by the initially colder material must equal the heat lost by the initially warmer material, according to the law of conservation of energy.
We know that when a substance absorbs heat energy, its temperature rises. When the same amount of heat is applied to equal masses of different substances, the temperature rise for each substance differs. Because different substances have varying heat capabilities, this is the case. So a substance’s heat capacity is the amount of heat required to raise the temperature of the entire substance by one degree. When the mass of a substance is one, the heat capacity is referred to as the specific heat capacity or the specific heat.
The amount of heat energy required to raise a substance’s temperature per unit mass is referred to as its Specific Heat Capacity. A substance’s specific heat capacity is a physical property. It is also an example of an extensive property because its value scales with the size of the system under consideration.
Overview:
In general, specific heat capacity is a measure of how much energy it takes to change a system’s temperature. However, it is vital to recognize that energy must be obtained by heating. The temperature will rise if work is done on the system; however, calculating the temperature rise using the heat capacity and the amount of work done on it is erroneous. Another thing to think about is the system’s constraints. Because the latter does work on its surroundings as it expands, the specific heat capacity of a constant volume system differs from that of a constant pressure system.
When working with solids, such differences are usually overlooked, but when working with gases, they are critical. The specific heat of a solid or liquid is the amount of heat required to raise the temperature of the solid’s unit mass by one degree Celsius. The letter C stands for it.
A substance’s specific heat capacity (symbol cp) is the heat capacity of a sample of the substance divided by its mass. Specific heat is also known as massic heat capacity at times. It is the quantity of heat that must be added to one unit of mass of a substance to generate a one-unit increase in temperature. Specific heat capacity is measured in SI units of joule per kelvin per kilogram J·kg-1·K-1.
When a substance, particularly a gas, is allowed to expand as it is heated (specific heat capacity at constant pressure), its specific heat capacity may be significantly higher than when heated in a sealed container to avoid expansion (specific heat capacity at constant volume). These two values are typically denoted by cp and cV respectively; their quotient =cp cV is the heat capacity ratio.
Molar Specific Heat:
The amount of heat energy required to raise the temperature of one mole of a substance is referred to as its molar heat capacity or molar specific heat capacity. Molar heat capacity in SI units is the amount of heat in joules required to raise one mole of a substance one Kelvin.
c n=Q/∆T
where Q denotes heat and ∆T denotes temperature change. For the most part, heat capacity is reported as an intrinsic property, which means it is a property of a specific substance. A calorimeter is used to measure heat capacity. For constant volume calculations, a bomb calorimeter is used. Coffee cup calorimeters are useful for determining constant pressure heat capacity.
Molar heat capacity is measured in J/K/mol or J/mol K units, where J is joules, K is Kelvin, and m is the number of moles. The value is based on the assumption that no phase changes occur. Typically, you’ll begin with the molar mass value, which is expressed in kilogrammes per mol. The kilogram-Calorie (Cal) or its cgs variant, the gram-calorie, is a less common unit of heat (cal). Heat capacity can also be expressed in terms of pound mass using temperatures in degrees Rankine or Fahrenheit.
The heat capacity per mole of atoms, or atom-molar heat capacity, is a closely comparable attribute of a substance in which the sample’s heat capacity is divided by the number of moles of atoms instead of moles of molecules.
The molar heat capacity of a substance, particularly a gas, can be much higher when the sample is allowed to expand while it is heated (at constant pressure, or isobaric) similar to the specific heat when heated in a closed vessel that prevents expansion (at constant volume, or isochoric). However, the heat capacity ratio determined from the matching specific heat capacities is the same for both.
A substance’s heat capacity dictates where and when it can be utilized. To protect the user’s safety, the handles and handles of utensils, for example, are made of materials with a high heat capacity. Thermometers, on the other hand, are made of materials with a low specific heat capacity, allowing them to detect even the tiniest temperature changes.
Definition Specific heat capacity:
The heat capacity C of a sample of a substance divided by the mass M of the sample is the specific heat capacity of the substance, generally written by c or s.
c=C/M
=1/M·dQ/dT
where dQ represents the amount of heat required to uniformly raise the temperature of the sample by a small increment, and dT represents the amount of heat required to uniformly Increase the sample’s temperature by a tiny amount.
The quantity of heat energy required to raise the temperature of a substance per unit of mass is known as specific heat capacity. A material’s specific heat capacity is a physical attribute. It’s also an example of a broad property because its worth is related to the size of the system under investigation.
Specific heat capacity is the amount of heat required to raise the temperature per unit mass. It’s usually the amount of heat in Joules required to increase the temperature of one gramme of material by one Kelvin or one degree Celsius. Water is ideal for temperature management because it has a high specific heat capacity.
Specific heat of water:
A liquid’s specific heat capacity (Cp) at normal temperature and pressure is approximately 4.2 J/g°C. This means that it takes 4.2 joules of energy to raise one degree Celsius from one gram of water. This Cp number is actually fairly high. This is the specific heat of the water as a liquid (1 cal/ g.deg) or the specific heat capacity of liquid water.
One calorie= 4.184 joules;
1 joule =1kg(m)²(s)-2=0.239005736 calorie
Water vapour has a higher specific heat capacity than most other materials at normal temperatures. The specific heat capacity (Cp) of water vapour at normal temperature and pressure is approximately 1.9 J/g°C. As with most liquids, water’s temperature rises as it absorbs heat and falls as it releases it. However, the temperature of liquid waterfalls rises more slowly than that of most other liquids. We can assert that water absorbs heat without increasing its temperature.
It also maintains its temperature for a much longer period of time than other substances. Similarly, hot water takes some time to cool down. As heat is released, the temperature drops and the vibrational activity of water molecules slows. The emitted heat compensates for the cooling effect of heat loss from the liquid water.
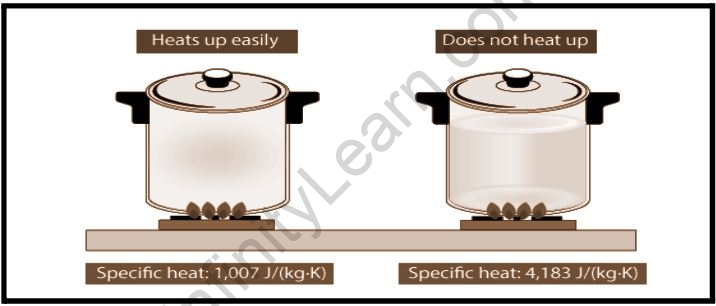
Some chemicals heat up quickly, while others take their time. Water is one of these; it has a high specific heat capacity because increasing the temperature requires more energy. The specific heat capacity of water is 4182 J/kg°C. We even have a particular way to define the amount of energy it takes to increase one gram of water by one degree Celsius, called a Calorie because water is such an important and common substance.
This is not the same as the calorie we talk about in food. This type of calorie is equal to 1,000 calories, which is why food-related calories are also known as kilocalories or kcals.
Conclusion
The hydrogen bonding can be used to explain why water has such high specific heat. Due to the multiple coupled hydrogen bonds, the molecules must vibrate in order to raise the temperature of the water. Because there are so many hydrogen bonds, it takes more energy to vibrate the water molecules and cause them to break. While molar heat capacity represents heat capacity per mole, specific heat capacity represents heat capacity per unit mass. Specific heat capacity is also abbreviated as “specific heat.” Volumetric heat capacity is sometimes used in engineering calculations instead of specific heat based on mass. The amount of heat required to raise the temperature of one mole of a substance by one Kelvin is referred to as its molar heat capacity. Because the joule is the SI unit of molar heat capacity, molar heat capacity is expressed in terms of J/molK. Specific heat capacity per unit mass is referred to as molar heat capacity.
Also read: Flow of Electric Charges in a Metallic Conductor
Frequently Asked Questions (FAQs):
How do you measure specific heat capacity?
The amount of heat energy required to raise one gramme of a product one degree Celsius is known as specific heat efficiency. The specific heat power of water is 4.2 joules per gramme per degree Celsius, or 1 calory per gram per degree Celsius.
Which is the advantage of water's heat capacity?
A one-degree increase in temperature needs more energy since water has a large heat capacity. The sun produces a continuous amount of energy, which causes sand and water to heat up faster.
What's the difference between heat capacity and specific heat capacity, and how do you calculate it?
The amount of heat required to raise a substance's temperature by one degree Celsius is known as specific heat capacity. Heat capacity, on the other hand, is the link between the quantity of energy provided to a substance and the resulting increase in temperature.
Why does water have a larger specific heat capacity than metal?
This is because the metal spoon's specific heat efficiency is substantially lower than that of the soup liquid. Water is the liquid with the highest specific heat capacity.








