Table of Contents
The International Union of Pure and Applied Chemistry (IUPAC) nomenclature of organic chemistry is a standard of naming organic chemical compounds which are approved in chemical nomenclature (IUPAC). It would be in the Journal of Organic Chemistry’s Terminology. Every potential organic compound should, in theory, have a name that can be translated into an unambiguous structural formula. Inorganic chemistry has its own IUPAC nomenclature. The official IUPAC naming rules are not often followed in practice, particularly where it is required to give a clear and absolute description to a compound, to avoid long and laborious names in routine communication. In certain cases, such as ethanol instead of ethyl alcohol, IUPAC nomenclature is simpler than earlier terms.
All compounds with carbon as the primary constituent are classified as organic compounds for nomenclature purposes. The three elements generally coupled with carbon to form the system of functional or characteristic groups are oxygen, hydrogen, and nitrogen. Other elements, such as halogens and sulfur, round out the basic elemental makeup of organic molecules. This style of nomenclature was so successful that it was expanded to include all elements in Groups 14, 15, 16, 17, and boron in Group 13; it might be extended to include all elements in Group 13.
A brief outline
The need for such a systematic method evolved as a result of the large number of novel organic compound discoveries, which made the trivial nomenclature of organic compounds extremely problematic. However, scientists may not always follow the IUPAC nomenclature recommendations since some compounds have excessively long and tiresome names as per the IUPAC nomenclature criteria. These compounds are given names that are more common. It’s worth noting that the availability of preferred IUPAC names doesn’t exclude the usage of other names to account for a particular context or to underline structural traits that are shared by a group of compounds. A “preferred IUPAC nomenclature” contains recommended IUPAC names.
All compounds with carbon as the primary constituent are classified as organic compounds for nomenclature purposes. The three elements generally coupled with carbon to form the system of functional or characteristic groups are oxygen, hydrogen, and nitrogen. Other elements, such as halogens and sulfur, round out the basic elemental makeup of organic molecules. Compounds containing this group of atoms were the first to be granted a substituted nomenclature.
Important concepts
IUPAC nomenclature
The nomenclature of compounds must follow these procedures, according to the IUPAC Guidelines:
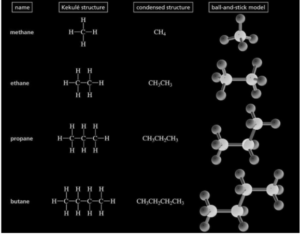
- The Longest Chain Rule dictates that the parent hydrocarbon be found and then named. The parent chain of the molecule in question is usually the longest carbon atom chain, whether it’s a straight-chain or a chain of any other shape.
- The Locants with the Fewest Numbers: The carbon atoms in the parent hydrocarbon chain must be numbered using natural numbers, starting at the end with the carbon atom that bears the substituents receiving the lowest number.
- There are several instances with the same substituent: The total amount of the same substituent in the provided chemical compounds is indicated by prefixes such as di, tri, etc.
- Naming of distinct substituents: Inorganic compounds with several substituents, the appropriate substituents are listed in the IUPAC nomenclature in alphabetical order.
- Various substituents at the same location are given different names: The substituents are named in ascending alphabetical order when two different substituent groups are present at the same location of the organic compound.
- Identifying and Naming Complex Substituents: Substituted alkyl groups must be given to complex substituents of organic compounds with branched structures, with the carbon linked to the substituent group being numbered as one. In the IUPAC nomenclature of the related compounds, these branching and complicated substituents must be written in brackets.
Methods of IUPAC Nomenclature
-
Nomenclature of Composition
In accordance with IUPAC nomenclature, compositional nomenclature is being used to name compounds depending on the makeup of the species or substances versus systems containing structural information or composition. The compositional naming of compounds uses the generalized stoichiometric term. Multiple prefixes are used to name substances so that the total stoichiometry of the compound can be determined from the name. When there are several components, they are split into two categories: electronegative components and electropositive elements.
-
Nomenclature of Substitutes
Substitutive Nomenclature has been used in the IUPAC nomenclature of compounds where hydrogen atoms are replaced with a substituent group to change the parent hydride. Organic compounds are named using functional groups as a prefix or suffix to the parent compound’s name in this naming system. This method can also be used to name compounds made from the hydrides of specific elements. These elements could also come from the ring- or chain-structured molecules.
-
Nomenclature Additive
This approach was created with the intention of being used in the nomenclature of coordination compounds. It would be used in a variety of ways. The name tri-chloride-phosphorus, which is used to designate the chemical with the formula PCl3, is an example of this nomenclature.
IUPAC name of acetic acid
The IUPAC term for acetic acid is the most often used and favored. The substitutive nomenclature is used to create the systematic name ethanoic acid, which is a legitimate IUPAC name. Acetic acid gets its name from the Latin word acetum, which means vinegar, and is linked to the word acid. Water-free (anhydrous) acetic acid is known as glacial acetic acid. The name stems from the ice-like crystals that form just below room temperature at 16.6 °C (61.9 °F), similar to the German word Eisessig (ice vinegar).
AcOH is a typical symbol for acetic acid, with Ac denoting the pseudo-element symbol for the acetyl group CH3C(=O) and Ac denoting the conjugate base, acetate (CH3COO). (The Ac is not to be confused with the sign for the element actinium; the context helps organic chemists distinguish between the two.) Acetic acid is written as CH3–C(O)OH, CH3C(=O)OH, CH3COOH, and CH3CO2H to better depict its structure. The abbreviation HAc is sometimes used in the context of acid-base reactions, where Ac is a sign for acetate in this example (rather than acetyl). Acetate is the ion formed when acetic acid loses its hydrogen ion. Acetate can also refer to a salt that contains this anion or an acetic acid ester.
IUPAC nomenclature Rules
Here’s a quick rundown of the rules to follow.
- Find the carbon chain with the longest length. This is regarded as the parent chain.
- Make a list of all the substitutes.
- From the end of the parent chain, count the carbons until the substituents have the lowest number. When comparing two sets of numbers, the “lowest” set is the one that has the lowest number at the time of the first difference. If two or more side chains are in the same place, give the one that will appear first in the name the lowest number.
- If the same substituent appears multiple times, the location of each spot where the substituent appears is indicated. A prefix also indicates the number of times the substituent group appears (di, tri, tetra, etc.).
- If there are two or more distinct substituents, they are listed alphabetically using the base name as the starting point (ignore the prefixes). When placing the substituents in alphabetically, the only prefix used will be iso, as in isopropyl or isobutyl. Except when compared to each other, the prefixes sec- and tert- are not employed in determining alphabetical order.
Significance of IUPAC nomenclature of organic compounds in NEET exam
Infinity Learn is the principal pick for some understudies with respect to NEET tests on account of the lucidity and legitimacy of the arrangements. We twofold check that the responses are right and that they will help all understudies. Understudies may quickly finish their Class 12 Physics schedule, which covers the strain part and its significant thoughts, with the assistance of Infinity Learn’s answers.
“Careful discipline brings about promising results,” as we’ve all heard. This is additionally valid for understudies in Class 12. Understudies can achieve outstanding imprints on their board tests, assuming they set what they’ve concentrated up as a regular occurrence. This not just permits understudies to recognize their solid and frail regions; however, it additionally assists them with having a less restless outlook on their tests.
Also read: Strength of Acid and Base
FAQs
Q. What is the significance of nomenclature?
Ans: The term “nomenclature” means the action of identifying things. It’s critical to be able to refer to it by a specific name. Nomenclature for living organisms is the scientific name. Working with various names for distinct species is critical, especially when working in the laboratory.
Q. In chemistry, what does nomenclature mean?
Ans: Choosing names for various science-related objects is known as nomenclature. Chemical nomenclature is defined as a set of rules or guidelines for producing or creating names for chemical substances. Chemical compounds frequently have a common name as well as a scientific name.
Q. Which of the following items is found in all organic molecules?
Ans: Despite the fact that all organic compounds contain carbon and virtually all of them contain hydrogen, the majority of them also contain other elements. The essential additional elements in organic molecules are oxygen, nitrogen, phosphorus, and halogens.







