Table of Contents
Halogen is a Greek word that means “salt producer.” Because chlorine, bromine, and iodine are highly electronegative in nature and form anions that make up the anionic part of salts found in seawater, they are referred to as salt producers. Astatine, the group’s final component, is radioactive in nature. All of these elements are found in the modern periodic table’s p block. The halogen family is the most homogeneous group in the modern periodic table after the alkali group. Halogens have high electronegativity, and the anions formed to become the anionic component of salts that are commonly found in seawater. The ability of an atom to accept an electron and form an octet is defined as electronegativity. Astatine, the group’s final component, is radioactive in nature.
The information about trends in chemical reactivity with halogens from various chemistry-related articles is available here. In nature, halogens are extremely reactive. They frequently form halides when they react with metals and nonmetals. Students who want to flourish in chemistry need to be well known about halogens and their chemical reactions to get deep knowledge about them to do well on their exams. The chemical reactivity of halogens, alkali metals and halogens reaction, and halogen displacement reactions colours are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional chemistry help.
Overview
In general, halogens are nonmetals in Periodic Table Group 17 (or VII). The size of the atoms decreases as one moves down the group. Fluorine has the weakest bond as a diatomic molecule due to repulsion between the electrons of the small atoms.
The boiling points of halogens rise as the strength of Van der Waals forces decreases in the group. As a result, the physical state of the elements lower in the group shifts from gaseous fluorine to solid iodine.
Halogens are highly electronegative due to their highly effective nuclear charge. As a result, they are extremely reactive and can gain an electron via reaction with other elements. When in sufficient quantities, halogens can be harmful or lethal to biological organisms.
Organic compounds containing halogen atoms are known as halogenated compounds or organic halides. Some halogens have multiple regulatory functions in the human body, while others are not required. The nucleophilic abstraction reaction is used to create organohalogens.
Polyhalogenated compounds are compounds that have been substituted with multiple halogens. Plenty of them are highly toxic and bioaccumulated in humans, but they have a wide range of potential applications.
Chemical Reactivity of Halogens
The ability of an atom to attract electrons or electron density towards itself within a covalent bond is referred to as electronegativity. The attraction between the nucleus and bonding electrons in the outer shell determines electronegativity. This, in turn, is determined by the balance of the nucleus’s number of protons, the distance between the nucleus and bonding electrons, and the shielding effect of inner electrons. The H-X bond in hydrogen halides (HX, where X is the halogen) grows longer as the halogen atoms grow larger. This means that the shared electrons are further away from the halogen nucleus, increasing the shielding of inner electrons. This means that electronegativity decreases as one moves down the group.
Halogens are said to be highly reactive elements that, in sufficient quantities, can be harmful or lethal to biological organisms. This reactivity can be attributed to high electronegativity and effective nuclear charge. Halogens can gain an electron by reacting with atoms of other elements.
One of the most reactive elements is fluorine. It reacts with inert materials such as glass and forms compounds with heavier noble gases. It is said to be a highly corrosive and toxic gas. Fluorine’s reactivity means that when it reacts with something, it forms such strong bonds that the resulting molecule is inert and non-reactive. In the presence of a small amount of water, fluorine can react with glass to form silicon tetrafluoride.
Trends in Chemical Reactivity with Halogens
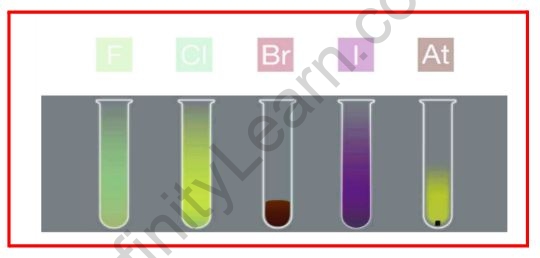
The physical properties of halogens vary in a very smooth manner. It is found that fluorine and chlorine exist as gases, bromine as liquids, and iodine as solids. The melting and boiling points of halogens rise as the element’s atomic number rises. The halogen family’s members are all coloured. This occurs due to the absorption of visible radiation, which causes electrons in the outer shell to be excited to higher energy levels. They absorb different quanta of radiation and thus appear in different colours. We have yellow fluorine, greenish-yellow chlorine, red bromine, and violet iodine. Water reacts with fluorine and chlorine. Bromine and iodine are only slightly soluble in water, but they are extremely soluble in other organic solvents such as chloroform and carbon disulphide.
The halogens have a -1 oxidation state in general, but chlorine, bromine, and iodine also have +1, +3, +5, and +7 states. Only when halogens combine with highly electronegative fluorine and oxygen atoms do they achieve a higher oxidation state. In nature, halogens are extremely reactive. They frequently form halides when they react with metals and nonmetals. As we move down the halogen family, the reactivity decreases. Halogens readily accept electrons because they are one electron short of forming an octet. As a result, they have a high oxidising capacity. Fluorine is the most powerful halogen oxidising agent, and it oxidises other halide ions in solution.
Alkali Metals and Halogens Reaction
Once alkali metals react with different halogens (Group 7 of the periodic table), a group of compounds known as alkali metal halides is formed. White solids are all alkali metal halides. Every alkali metal reacts violently with halogens to form salts, the most important of which are NaCl and KCl.
2Na(s)+Cl2(g)→2NaCl(s)
Sodium chloride is used as a meat preservative and to melt ice on roads (via freezing point depression). Because potassium has a positive effect on plant growth, KCl is important in plant fertilizers. Except for LiF, which has a high lattice enthalpy due to strong electrostatic attraction between Li+ and F– ions, all of these metal halides form white ionic crystalline solids and are all soluble in water.
Some examples are:
| lithium | + | [halogen] | lithium | [halide] | |
| chlorine | chloride | ||||
| bromine | bromide | ||||
| iodine | iodide | ||||
| sodium | + | [halogen] | sodium | [halide] | |
| chlorine | chloride | ||||
| bromine | bromide | ||||
| iodine | iodide | ||||
| potassium | + | [halogen] | potassium | [halide] | |
| chlorine | chloride | ||||
| bromine | bromide | ||||
| iodine | iodide | ||||
Halogen Displacement Reactions Colours
To a lesser extent, halogens react with water, forming acidic solutions with bleaching properties. In solution, they also undergo redox reactions with metal halides, displacing less reactive halogens from their compounds. These displacement reactions are used to determine the reactivity order down Group 17 of the periodic table.
Once chlorine (as a gas or dissolved in water) is added to a solution of sodium bromide, the chlorine replaces the bromine. Chlorine displaces bromine from sodium bromide because it is more reactive than bromine.
The solution darkens. This brown colour is caused by bromine that has been displaced. The chlorine has been converted into sodium chloride.
chlorine + sodium bromide sodium chloride + bromine
Cl2 (aq) +2NaBr (aq) →2NaCl (aq) +Br2 (aq)
One such type of reaction occurs for all halogens. In a solution of one of its salts, a more reactive halogen displaces a less reactive halogen.
Also read: Friedel Craft’s Alkylation and Acylation
FAQs
Why do halogens exhibit colour?
In different regions, halogens absorb the visible range's colour, causing valence electrons to become excited and jump to higher energy states. Thus, fluorine has a yellow colour, bromine has a red colour, chlorine has a greenish-yellow colour, and iodine has a violet colour.
Why does chlorine have a higher electron affinity than fluorine?
Fluorine has a quite small size and thus is compact. As a result, the crowded electrons in fluorine have a screening effect, which can impede electron acceptance. However, because chlorine is larger than fluorine, it can easily accommodate an electron. As a result, chlorine has a greater electron affinity than fluorine.
Explain why halogens are more reactive.
To achieve the octet of a noble gas configuration, halogens only need to gain one electron. Furthermore, because they are the smallest atoms in a period, they have a high electronegativity (attraction for electrons) and tend to attract electrons in a bond. As a result, they can form bonds with any other metal or electropositive ion with ease. Halogens tend to attract shared pairs of electrons in a covalent bond.








