Table of Contents
The Friedel–Crafts reactions were invented in 1877 by Charles Friedel and James Crafts to attach substituents to aromatic rings. There are two types of Friedel–Crafts reactions: alkylation reactions and acylation reactions. Both are accomplished through electrophilic aromatic substitution. The Friedel-Crafts reaction is an aromatic chemical reaction in which an electrophilic aromatic substitution occurs. This well-known reaction was invented by two scientists, French Charles Friedel and American James Crafts. In the Friedel-Crafts reaction, the aromatic compound undergoes an electrophilic substitution. The hydrogen atom in benzene is replaced by an electrophile in the presence of a Lewis acid, such as anhydrous aluminium chloride. Friedel-Crafts Reactions are classified into two types:
- Alkylation by Friedel-Crafts
- Acylation by Friedel-Crafts
Electrophilic aromatic substitution occurs in both types of Acetyl Chloride Benzene ions, Friedel craft alkylation, and Friedel craft acylation.
Overview
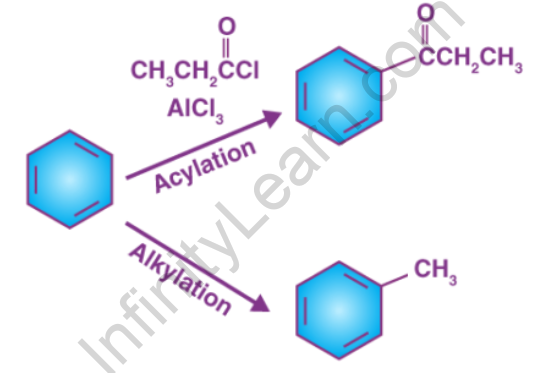
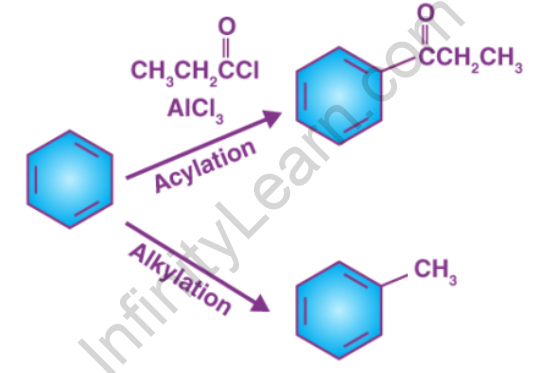
The alkylation of an aromatic ring is involved in Friedel–Crafts alkylation. Alkyl halides have traditionally been used as alkylating agents. Alkylating agents other than alkyl halides can be used. Enones and epoxides, for example, can be used in the presence of protons. Traditionally, a strong Lewis acid, such as aluminium chloride, is used in the reaction. Because alkyl groups are activators for the Friedel–Crafts reaction, the product is more nucleophilic than the reactant in this reaction. As a result, overalkylation can occur. As in the t-butylation of 1,4-dimethoxybenzene, steric hindrance can be used to limit the number of alkylations.
The acylation of aromatic rings is involved in Friedel–Crafts acylation. Acyl chlorides are common acylating agents. Acid anhydrides and carboxylic acids are also viable options. Aluminium trichloride is a common Lewis acid catalyst. However, because the product ketone forms a relatively stable complex with Lewis acids such as AlCl3, a stoichiometric amount or more of the “catalyst” must generally be used, as opposed to the Friedel–Crafts alkylation, which requires constant regeneration of the catalyst. The reaction conditions are comparable to those of the Friedel–Crafts alkylation. This reaction has a number of advantages over alkylation. Because of the carbonyl group’s electron-withdrawing effect, the ketone product is always less reactive than the original molecule, so multiple acylations do not occur.
Mechanism of Friedel crafts alkylation
Friedel-Crafts alkylation is the substitution of an aromatic proton for an alkyl group. This is accomplished through an electrophilic attack on the aromatic ring using carbonation. Alkyl halides are used as reactants in the Friedel-Crafts alkylation reaction to produce alkylbenzenes. The Friedel-Crafts alkylation of benzene is depicted below.
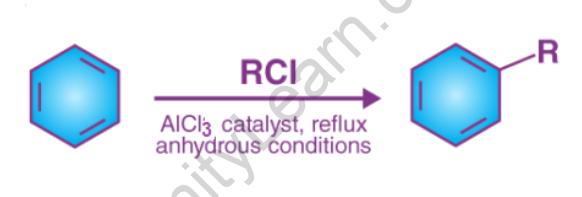
A Lewis acid catalyst, such as FeCl3 or AlCl3, is used in this reaction to facilitate the removal of the halide and thus form a carbocation.
Before proceeding with the alkylation reaction, the resulting carbocation undergoes a rearrangement.
Mechanism
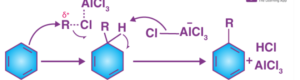
The Friedel-Crafts alkylation reaction is a three-step process.
Step 1: The Lewis acid catalyst (AlCl3) reacts with the alkyl halide, forming an electrophilic carbocation.
Step 2: The carbocation attacks the aromatic ring, forming an intermediate cyclohexadienyl cation. Because of the breakage of the carbon-carbon double bond, the aromaticity of the arene is temporarily lost.
Step three: The intermediate’s deprotonation causes the carbon-carbon double bond to reform, restoring the compound’s aromaticity. This proton then reacts with water to form hydrochloric acid, regenerating the AlCl3 catalyst.
Mechanism of Friedel crafts acylation
The addition of an acyl group to an aromatic ring is the Friedel-Crafts acylation reaction. This is typically accomplished by using an acid chloride (R-(C=O)-Cl) and a Lewis acid catalyst such as AlCl3. The aromatic ring is converted into a ketone in a Friedel-Crafts acylation reaction. Under these conditions, the reaction between benzene and an acyl chloride is depicted below.
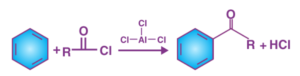
In Friedel-Crafts acylations, an acid anhydride can be used instead of an acyl halide. The acyl halide’s halogen forms a complex with the Lewis acid, resulting in a highly electrophilic acylium ion with the general formula RCO+ that is stabilized by resonance.
Mechanism
Friedel-Crafts acylations are accomplished in four steps.
Step 1: a reaction takes place between the Lewis acid catalyst (AlCl3) and the acyl halide. A complex is formed, and the acyl halide loses a halide ion, resulting in the formation of an acylium ion that is stabilized by resonance.
Step 2: The acylium ion (RCO+) then attacks the aromatic ring electrophilically. As a complex form, the aromaticity of the ring is temporarily lost.
Step 3: The intermediate complex is now deprotonated, restoring the ring’s aromaticity. This proton binds to a chloride ion (from the complexed Lewis acid) to form HCl. The AlCl3 catalyst has now been rejuvenated.
Step 4: Now that the catalyst has been regenerated, it can attack the carbonyl oxygen. As a result, the ketone product must be liberated by combining the products formed in step 3 with water.
As a result of the Friedel-Crafts acylation reaction, the required acyl benzene product is obtained.
Riedel crafts acylation of benzene mechanism
The electrophilic aromatic substitution is at the heart of the Acylation reaction’s mechanism. As previously stated, acylation is the process or mechanism of adding an acyl group (RCO-) to a specific compound in Organic Chemistry. The reaction entails a nucleophile (electron donor) substituting at the electrophilic carbonyl group (Cgraphic image 3O) of a carboxylic acid derivative. In most cases, the substitution is accomplished through an addition-elimination reaction. While there are numerous acylating reagents, the two most common are acid halides (X = Cl, Br) and anhydrides (X = OCOR). Alcohols (ROH) or phenols are the nucleophiles in the acylation reaction (ArOH). They both produce esters, ammonia, and amines. Acyl halides are commonly used as acylating agents because they form strong electrophiles when combined with certain metal catalysts.
FAQs
What is Friedel Craft's reaction in this case?
An alkyl group can be added to a benzene molecule via an electrophile aromatic substitution reaction known as the Friedel-Crafts alkylation reaction. One example is the addition of a methyl group to a benzene ring. The electrophile attacks the electron system of the benzene ring to form a nonaromatic carbocation.
What are the benefits of acylation by Friedel Crafts?
Friedel-Crafts acylation has a few advantages over alkylation because it uses a Lewis acid catalyst and an acyl chloride to add benzene to an acyl ring. The ketones produced can be reduced to alkyl groups using Clemmensen reduction.
What exactly is benzene alkylation?
Alkylation is the process of substituting an alkyl group for something, in this case, a benzene ring. A hydrogen ring is substituted by a group such as a methyl or ethyl, for example. Benzene is treated with chloroalkane in the presence of aluminium chloride as a catalyst.



