Table of Contents
Carbocations and Carbanions
Carbocation and Carbanion are terms used to describe organic chemical entities that have an electrical charge on a carbon atom. The primary distinction between carbocation and Carbanion is that carbocation has a positively charged carbon atom, whereas Carbanion contains a negatively charged carbon atom.
Carbocation:
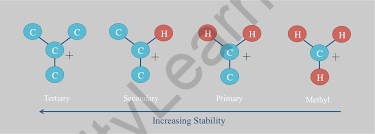
- A carbocation is a molecule with a positively charged carbon atom and three bonds. We can term them carbon cations. It was previously known as carbonium ion. A carbocation is any even-electron cation with a large positive charge on the carbon atom.
- Carbon’s hybridization in carbocation will be sp2, and its form will be trigonal planar. There is also an empty p orbital, indicating that it is electron-deficient. Carbon’s valence shell has six electrons. As a result, it is an electron-deficient species known as an electrophile.
- A carbocation is commonly observed in an SN1 reaction, an elimination reaction, and so on.
- In chemical processes, carbocation acts as an electrophile.
Carbanion:
- A carbanion is a negatively charged ion in which a carbon atom demonstrates trivalence (i.e., it forms three bonds) and has a formal negative charge with a value of at least -1.
- Carbanions often have a bent, straight, or trigonal pyramidal molecular geometry when pi delocalization does not occur in the organic molecule (as in aromatic compounds).
- It should be noted that all carbanions are conjugate bases of certain carbon acids.
- In Carbanion, the carbon atom with the negative charge is sp3 hybridized.
- In chemical processes, Carbanion acts as a nucleophile.
In contrast to carbocations and carbon radicals, electron-donating groups attached to the anionic core destabilize a carbanion because the centre already possesses an octet of electrons. As a result, the order of stability of carbanions is the inverse of that of carbocations and radicals.
Carbocations and carbanions are often encountered as intermediates in a variety of processes.
Get the most Important Questions in Physics, Chemistry, math, and Biology.
FAQs
Explain the Factors Affecting the Carbanion's Stability.
An anion is a carbanion when the carbon is tervalent, makes three bonds, and has a formal negative charge in the one important mesomeric contribution. The stability is determined by the inductive hybridization impact of the anion's conjugation charge extent. The C-C of numerous bonds and neighbouring carbon atom lone pairs are two characteristics that influence Carbanion. A carbocation is positively charged, and as the number of positively charged atoms falls, so does the stability of carbon.
What effect does electronegativity have on carbocation stability?
Electronegativity is an atom's capacity to attract electrons. The greater the Electronegativity, the greater the electron attraction to the atom. The Electronegativity of the positively charged carbon directly affects the stability of the carbocation. The carbocation's stability reduces as the carbon atom's Electronegativity rises. Sp> sp2 > sp3 The hybridization of the positively charged carbon in the vinylic carbocation is sp, which has a higher electronegativity than the hybridized carbon in the sp2 alkyl carbocation. As a result, a primary vinylic carbocation has lower stability than the main alkyl carbocation.
Infinity Learn App
Now you can find answers to all your subject queries & prepare for your Exams on our Ultimate Learning App for Classes 6 to 12 – Infinity Learn.






