Table of Contents
Reactants and products coexist in equilibrium, resulting in a less than 100% reactant-to-product conversion rate. In polar solvents, equilibrium reactions might entail the breakdown of a non-polar (covalent) reactant or the ionization of ionic molecules into their ions.
We’ll study ionic equilibrium in ionic solutions in this section. On the basis of their capacity to conduct electricity, substances in ionic equilibrium can be divided into two types, as shown below.
Non-Electrolytes
These are molecules that have no electric charge, do not dissolve into their constituent ions, and hence do not conduct electricity in an aqueous solution or in molten form. Consider a sugar solution.
Electrolytes
These are compounds that in their aqueous solution dissociate into their constituent ions and hence conduct electricity in their aqueous solution or molten form. The salt solution, acid solution, base solution, and so forth.
In ionic equilibrium, there are two types of electrolytes: strong and weak electrolytes.
Strong electrolytes are chemicals that ionize entirely when dissociated in their ionic solution, whereas weak electrolytes only dissociate partially.
For example, in its aqueous solution, NaCl undergoes full ionization, yielding sodium ions (Na+) and chloride (Cl–) ions, whereas acetic acid experiences partial ionization, yielding some acetate ions (CH3COO–) and hydrogen (H+) ions.
1. The dissociation reaction is considered to be complete in the case of a strong electrolyte, going only forward, but the reaction is said to be reversible in nature in the case of a weak electrolyte.
2. Ionic equilibrium is formed in the case of a weak electrolyte between the ions and the unionized molecules.
Formulas for Ionic Equilibrium
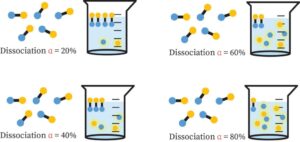
At equilibrium, it’s important to know what proportion of the reactants’ initial amount is changed into products.
The degree of dissociation/ionization refers to the percentage of the starting molecules that are transformed at equilibrium.
(Number of reactant molecules dissociated ionized at the start)/ (Degree of dissociation or ionization) (Number of reactant molecules at the start)
In ionic equilibrium, the degree of dissociation can be stated as a percentage.
% Dissociation or ionization = (Number of reactant molecules dissociated or ionized at the start)/(Number of reactant molecules at the start) 100.
Also read: Important Topic of Chemistry: Covalent Bond
FAQs
Q. What is Ionization Percentage?
Ans: The degree of ionization is determined by a variety of factors.
1. Electrolyte nature: strong, weak, or insoluble
2. Solvent nature: Solvents with a high dielectric constant promote ionization.
3. Dilution: the higher the dilution, the more ionization occurs.
4. Temperature: as the temperature rises, so does the ionization.
5. The ionization of the weak electrolyte is reduced when common ions are present.
Is there a difference between ionization and dissociation?
Ionization and dissociation are two terms that refer to the same thing: the separation of elements. Ionization and dissociation are distinguished by the fact that ionization always produces electrically charged particles, whereas dissociation may or may not produce electrically charged particles.
What happens when water ionizes or dissociates into ions?
Dissociation occurs when ionic substances dissolve in water and their ions separate from one another. Water, like many other covalent compounds, may dissociate into ions, which is a fascinating characteristic.