Table of Contents
Introduction:
It is considered a standard lab technique for deciding the sub-atomic load of an unstable fluid. This idea was found by Victor Meyer. In this cycle, a known mass of unstable fluid or strong is put under assessment and they are changed over into its fume structure by warming by utilizing Victor Meyers tube, as this cylinder helps in the transformation of unpredictable strong or fluid into fume structure. Fume helps in the removal of its own volume of air and the volume of air uprooted at a given trial temperature and tension is determined. At STP ( standard temperature pressure ) i.e at 2.24×10−2, dislodged mass of air is determined. The worth which is acquired from this interaction is known as the sub-atomic mass of the substance.
The fact of Determining Molecular Masses utilizing Victor Meyer’s Method
- To figure out the overall sub-atomic mass of obscure fluid abuse of the Victor Meyer hardware.
- The Victor Meyer method contains disintegrating a notable load of fluid in during an incredibly in a chamber kept up with at an adequate and consistent outrageous temperature. The air dislodged from the chamber by the disintegrated test is cooled to temperature, and its volume is thoroughly estimated. Replacement of air for the specific fume, in this way, gives an approach to concluding the sum the notable mass of fume would possess at a temperature in the event that it very well may be cooled while not consolidating.
Development
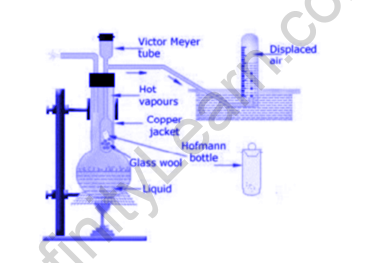
- The instrumentation of this interaction incorporates an internal Victor Meyer’s cylinder, the lower end of which is as a bulb. The upper finish of the cylinder has a side cylinder that prompts a box loaded up with water. The cylinder which is known to be Victor Meyer’s cylinder is encircled by a coat from outside and fluid is put in an external coat, which as a rule bubbles somewhere around 30k more than the substance when held under assessment. Utilization of Meyers tube is utilized to shield the instrument from external breakage when a glass bottle containing the substance under assessment is dropped to it.
- An aspherical base jar brimming with a fluid whose B.P. is 10c over the unstable fluid goes about as a partner degree external glass coat. A victor Mayor’s cylinder with a partner degree external cylinder goes about as an internal glass coat. This external cylinder is lordotic in a very box fitted with water. The extremely modest Victor Mayor’s cylinder comprises of Hg or amphibole things for cushioning.
- The external glass coat is warmed on account of the that air grows and rises through the water inside the box. A little low glass tube is considered Hoffman’s container with a plug that is spotless, washed and dried, and weighted. The unpredictable fluid is taken in Hoffman’s jug and weighted. An estimating instrument tube loaded with water is set over the cylinder associated with victor common power gear.
- Presently Hoffman’s jug is brought into the world inside the Victor common power tube. On account of hotness, fluid inside the container dissipates and passes over the plug and dislodges air that relates to volume is gathered in a very estimating instrument tube by descending removal of water.
- The water temperature and strain are noted. The estimating instrument tube is taken into another box brimming with water for levelling of strain. The amount of air dislodged is noted, which relates to the number of fumes.
Mathematical Representation of Victor Meyer Method
- This strategy is utilized for the assurance of the sub-atomic mass of unstable natural mixtures. Under this cycle, we take the known mass of the compound, and this compound is disintegrated in an instrument known as Victor Meyer tube. How many fumes got is estimated and diminished to standard temperature pressure i.e STP.
Let the volume of fumes got at STP be V mL
22400 mL of fumes are acquired from 1 mole of a given compound.
V mL of fumes are gotten from (V/22400) mL of the compound
Mole= W/Mw
Accordingly,
W/Mw = V/22400
Uses of Victor Meyer Method
1. Distinguishing proof of Alcohols
- This strategy is utilized for the ID of liquor. From this, you can decide essential, optional, and tertiary alcohols by an adjustment of their shadings. During this cycle, liquor is treated with Phosphorus triiodide which is additionally treated with Silver nitric oxide to get nitroalkane as an item. Further, this item i.e nitroalkane is treated with nitrous corrosive which is gotten by the response of NaNO2 and HCl. The last wrestling arrangement is treated with KOH and the necessary tone is gotten. On the off chance that it is red the shading shows essential liquor, in the event that blue it demonstrates optional liquor and assuming no shading is seen, demonstrates tertiary liquor.
- Alcohols are the hydroxyl subordinates of alkanes which contains – OH useful gathering. These mixtures are generally acquired from the alkanes subsequent to supplanting the hydrogen iotas with a hydroxyl bunch. A liquor that contains two-OH bunches is called Dihydric liquor.
2. Assurance of the Empirical Formula and the Molecular Formula
- This strategy is utilized for the assurance of both exact as well as sub-atomic recipes, by utilizing the given technique. An exact equation lets us know the general proportions of various iotas in a compound.
- Though to observe an atomic equation, we want to know the sub-atomic mass of the given compound.
What is Molecular Formula?
- The sub-atomic recipe is gotten from the particles in a molecule. It addresses the absolute number of individual particles present in an atom.
- The anatomic equation additionally has an addendum. This addendum reports the real number of each kind of particle in an atom of the compound.
- The Molecular recipe is likewise connected with gram atomic masses. These are basic entire Numbers of the comparing experimental recipe mass.
What is an Empirical Formula?
- The observational equation is the most straightforward recipe for a compound. The observational equation is characterized as the proportion of addendums of the littlest conceivable entire number of the components present in the recipe. It is by and large the easiest known equation for a compound.
- An observational equation for a compound is the recipe of a compound composed with the littlest whole number addendum.
- The observational equation gives data about the proportion of quantities of molecules in a compound. The per cent organization of a compound straightforwardly prompts its observational recipe.
- The connection between the sub-atomic equation and the exact recipe is
Sub-atomic Formula = n x Empirical equation
Empirical Formula
- An exact equation addresses the most straightforward entire number proportion of different particles present in a compound.
- Model: the observational recipe of acetylene is CH
Molecular Formula
- The sub-atomic equation is the portrayal of the specific number of various sorts of particles present in a particle of a compound.
- Model: For Acetylene the observational equation is C2H2
FAQs
What are the benefits of Vector Mayer's strategy?
The strategy is exceptionally easy to do the weight. The delineation expected for the investigation is tiny.
What are the bad marks of Vector Mayer's Method?
This strategy applies to unpredictable fluid as it were. The strategy can not be utilized for the substance which goes through warm disintegration.
How would you decide atomic load by Victor Meyer's technique?
Victor Mayer tube is utilized to disintegrate a known mass of the compound. The fumes are applied from the example and it dislodges an equivalent number of filled air from it into a graduated cylinder. The deliberate volume of fumes is diminished to STP.