Table of Contents
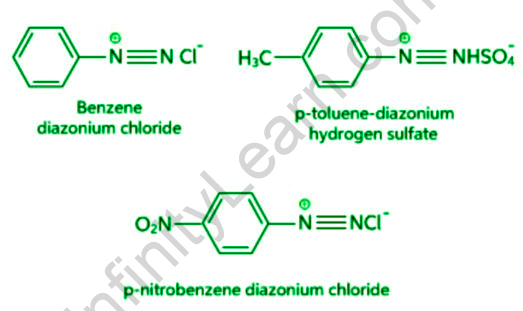
Any of a class of chemical compounds with the molecular formula diazonium. R is an atomic grouping formed by removing a hydrogen atom from the structural formula of an organic compound. Diazonium salts are formed by reacting primary amines with nitrous acid (diazotization); their most distinguishing feature is their instability. Aliphatic diazonium salts exist only as brief intermediates, quickly dissolving into a nitrogen molecule and a carbonium ion; certain aromatic diazonium salts are stable enough to isolate, but readily react with nitrogen loss or the formation of azo compounds. Diazonium salts were first synthesized in 1858 from aromatic amines, and the dye industry quickly recognized their utility in the production of azo compounds. Colours from the visible spectrum are added to dyes suitable for a variety of fibres by modifying the chemical structures of the diazotized amines (the diazo components) and the compounds with which they react (the coupling components). The diazonium group can be replaced by a variety of atoms or groups of atoms, often with the assistance of copper or a copper salt; these reactions allow for the preparation of a wide range of aromatic derivatives. Hydrazine derivatives are formed through the chemical reduction of aromatic diazonium salts.Diazonium compounds, also known as diazonium salts, are a class of organic compounds that share the functional group R−N+2X− , where R can be any organic group, such as an alkyl or an aryl, and X can be an inorganic or organic anion, such as a halogen.
For instance, Halogen, Chlorine, Bromine, and so on. Diazonium compounds are another name for diazonium salts. We can make phenols by heating it in water. Furthermore, azo compounds can be synthesized by reacting diazonium salts with other aromatic compounds.
Overview
Diazonium salts are organic compounds with the formula R–N2+X–, where R can be any alkyl or aryl group and X can be halogens, hydrogen sulphate, or other organic compounds.
Aryl diazonium salts are frequently used as chemical intermediates. The diazonium group is easily replaced by a variety of functional groups, including –I, –OH, –F, –CN, and –H, which cannot be substituted directly into the aromatic ring. Furthermore, replacement patterns that are diametrically opposed to the norm can be generated (i.e., preparation of 1,3-dihalo substituted benzenes). The diazonium salt is not separated in the majority of these replacements.
Arenediazonium salts are a type of chemical that contains arene diazonium. The word di refers to two, aza to nitrogen, and onium to the ionic nature of the compound in the phrase “Diazonium salts.” As a result, diazonium salts are ionic compounds that contain N≡N. Diazonium salts are organic compounds that have triple bonds between Nitrogen atoms and either an alkyl or an aryl (benzene ring) on the other side. Diazonium salts are the transitional phase between azo dyes (or compounds are known to be popular colouring agents). Salts are named after the double nitrogen (diazo) found in ionic salts, where chloride molecules replace the nitrogen atom.
Chemical reactions of Diazonium salts
Diazonium salts are subjected to two types of reactions:
- Reactions involving the substitution of nitrogen
- Reactions involving the retention of the diazo group
This article discusses the reactions that involve the substitution of nitrogen. Diazonium is an excellent leaving group. Other groups, such as Cl–, Br–, CN–, and OH–, easily displace nitrogen from the aromatic ring. This nitrogen escapes as a gas from the reaction mixture.
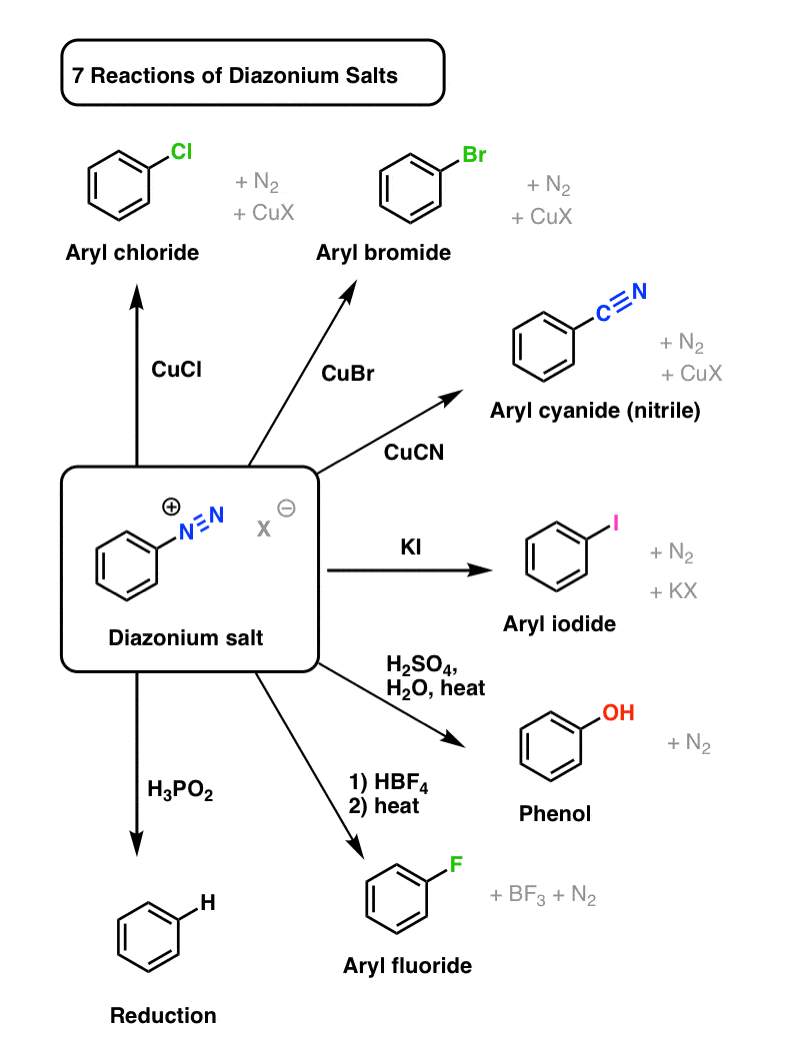
1) Replacement by halide or cyanide ion: In the presence of Cu (I), nucleophiles such as Cl–, Br–, and CN– can easily enter the benzene ring. This is commonly referred to as the Sandmeyer reaction.
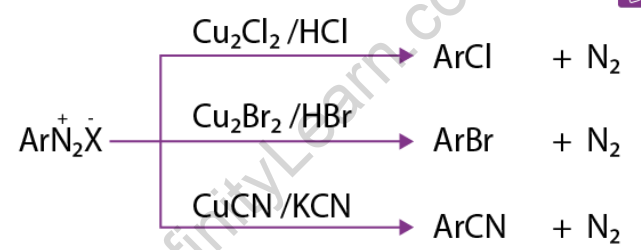
2) Iodide ion replacement: It is difficult to introduce iodine directly into the benzene ring. Iodobenzene is formed when diazonium salt solution is treated with potassium iodide.

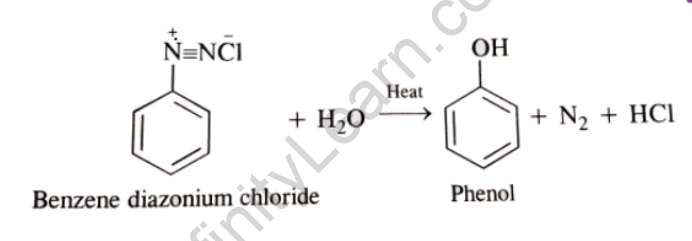
3) When the temperature of the diazonium salt solution is raised to 283 K, the salt is hydrolyzed to phenol.
4) Replacement by –NO2 group: When diazonium fluoroborate is heated in the presence of copper with aqueous sodium nitrite solution, the diazonium group is replaced by –NO2.
Benzene diazonium chloride reactions
We can divide benzenediazonium chloride reactions into two categories.
- Reactions caused by the substitution of one group for the diazonium group
- Diazonium ion coupling reactions
Benzene diazonium chloride is a raw material used in the manufacture of dyes. In the following section, we will look at those dye preparation reactions as well.
Reactions caused by the substitution of one group for the diazonium group
Benzene Diazonium chloride (C6H5-N2Cl) can be converted into a variety of important aromatic organic compounds, including chlorobenzene, bromobenzene, phenol, and others. The -N2Cl group is replaced with another group such as -Cl, -Br, -OH, and so on.
Benzene diazonium chloride Sandmeyer reactions
Traugott Sandmeyer, a Swiss chemist, discovered SandMeyer reactions in 1884. As a result of Sandmeyer reactions, we can produce chloribenzene, bromebenzene, benzonitrile, iodobenzene, and flurobenzene. When benzenediazonium chloride is treated with CuCl (Copper(I) chloride), chlorobenzene is formed. Instead of CuCl, copper powder with HCl can be used. Cu+ ion acts as a catalyst in this reaction. When benzenediazonium chloride is treated with CuBr (Copper(I) bromide), bromobenzene is formed. Instead of CuBr, copper powder with HBr can be used. Cu+ ion acts as a catalyst in this reaction.
Benzonitrile is formed when benzenediazonium chloride is treated with CuBr (Copper(I) bromide). The reaction of benzenediazonium chloride and KI can produce iodobenzene (potassium iodide). However, the reaction medium must be heated. As with the previous three reactions, a Cu+ catalyst is not required for this reaction.
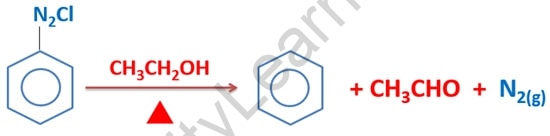
This reaction produces benzene, acetaldehyde, and nitrogen gas as byproducts. As you can see, ethanol is oxidized to produce acetaldehyde, an aldehyde compound.
Diazonium reactions
The salts of aryl diazonium are important intermediates. They are prepared in a cold (0 o to 10 oC) aqueous solution and generally react with nucleophiles with nitrogen loss. The diagram below depicts some of the more commonly used substitution reactions. These reactions are energetically favoured because the leaving group (N2) is thermodynamically very stable. Sandmeyer reactions are substitution reactions that are catalyzed by cuprous salts. Fluoride substitution occurs when BF4(–) is added, a reaction known as the Schiemann reaction. Stable diazonium tetrafluoroborate salts can be isolated, and when heated, the nitrogen is lost, yielding an arylfluoride product. The most notable reaction with hypophosphorus acid, H3PO2, is the reductive removal of an amino (or nitro) group.
FAQ’s
What exactly are diazotization and reaction?
Aromatic amine reacts with nitrous acid and mineral acid to form diazonium salt, which generates water as a byproduct. This is known as the Diazotization Reaction.
What reaction is the diazonium salt involved in?
A diazonium salt is formed when amine reacts with nitrosonium ion. Because diazonium salt is usually unstable, it is usually made in situ. When the alkyl group is replaced with an aryl group, the salt is stable at 0 degrees Celsius and reacts with a variety of nucleophiles. Tiffeneau-Demjanov rearrangements employ the diazonium salt.






